Introduction: Electrospun fibers have been widely explored as drug delivery systems for physicochemically diverse drugs[1]. Sustained release remains a formidable challenge from these materials, which have a large surface area conducive to rapid diffusive transport[2]. Hydrophobic polymers can sustain drug release from fibers[3], but are typically slow- or non-biodegradable. The ability to tune the degradation while maintaining sustained release of small molecule drugs will be important for many medical applications. Segmented polyurethanes (PUs) can be designed to be biodegradable, nontoxic[4],[5] and allow for variance of hard-to-soft segment ratio or pendant groups[6]. We investigated the release of physicochemically diverse drugs from fibers electrospun with a new class of biodegradable PUs that incorporate PLGA-b-TEG-b-PLGA copolymers (PLGA-PU). Our PLGA-PUs show more rapid degradation than PUs based on polycaprolactone. We show that varying the hard segment and fluorobenzene content can modulate drug release[7].
Materials and Methods: PLGA-PUs were synthesized from PLGA-(TEG)-PLGA diol (PolySciTech, MW=1260 Da), or lysine diisocyanate (LDI) (Kyowa Hakko USA), 1,4-butanediamine (BDA) (Sigma), 1,3-diamino-2-hydroxypropane (DAHP) (Sigma) and 4-fluorophenyl isocyanate (FPI) (Sigma) in the molar ratios indicated in Figure 1. PUs were characterized for fluorine, molecular weight and hydrophobicity using 19F-NMR, GPC and goniometry. Polymers were dissolved in DCM or HFIP and electrospun using a needle rig at 10 cm and 15 kV with a flow rate of 30 μL min-1. Fibers were loaded with 15 wt.% levonorgestrel (LNG), maraviroc (MVC), norfloxacin (NFL) or tenofovir (TFV) and characterized for fiber size and drug crystallinity using SEM and DSC. Release studies were carried out in 2% solution in water at 37°C and analyzed using HPLC.
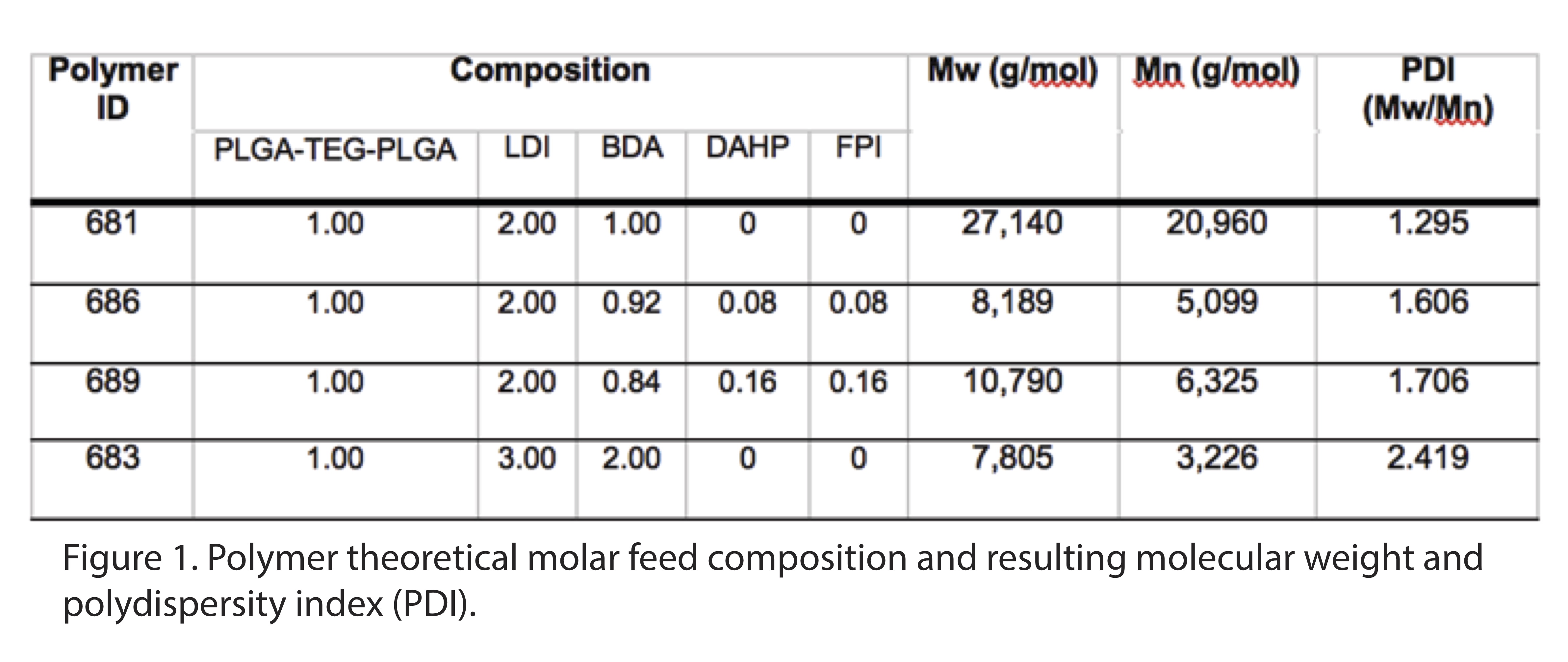
Results and Discussion: We synthesized PLGA-PUs with either variable hard segment or fluorobenzene pendant content. Our PLGA-PUs had an average MW of 8-27 kDa (Figure 1) and could be electrospun to yield uniform and smooth fibers. While PCL-PU reported in the literature degrade 10-25%[8], our PLGA-PU showed 65-80% degradation within 4 weeks. By increasing the hard-to-soft segment ratio by two-fold, we augmented the sustained release of the most hydrophilic drug, TFV, but not the most hydrophobic drug, LNG (Figure 2). In contrast, by increasing the molar content of a fluorobenzene pendant by two-fold, we were able to modulate release of LNG, but not TFV. Finally, polymer hydrophobicity increased with an increased hard segment and fluorobenzene content. By modifying the composition of these novel PLGA-PUs, we are able to sustain release of physicochemically diverse drugs without altering the degradation rate.
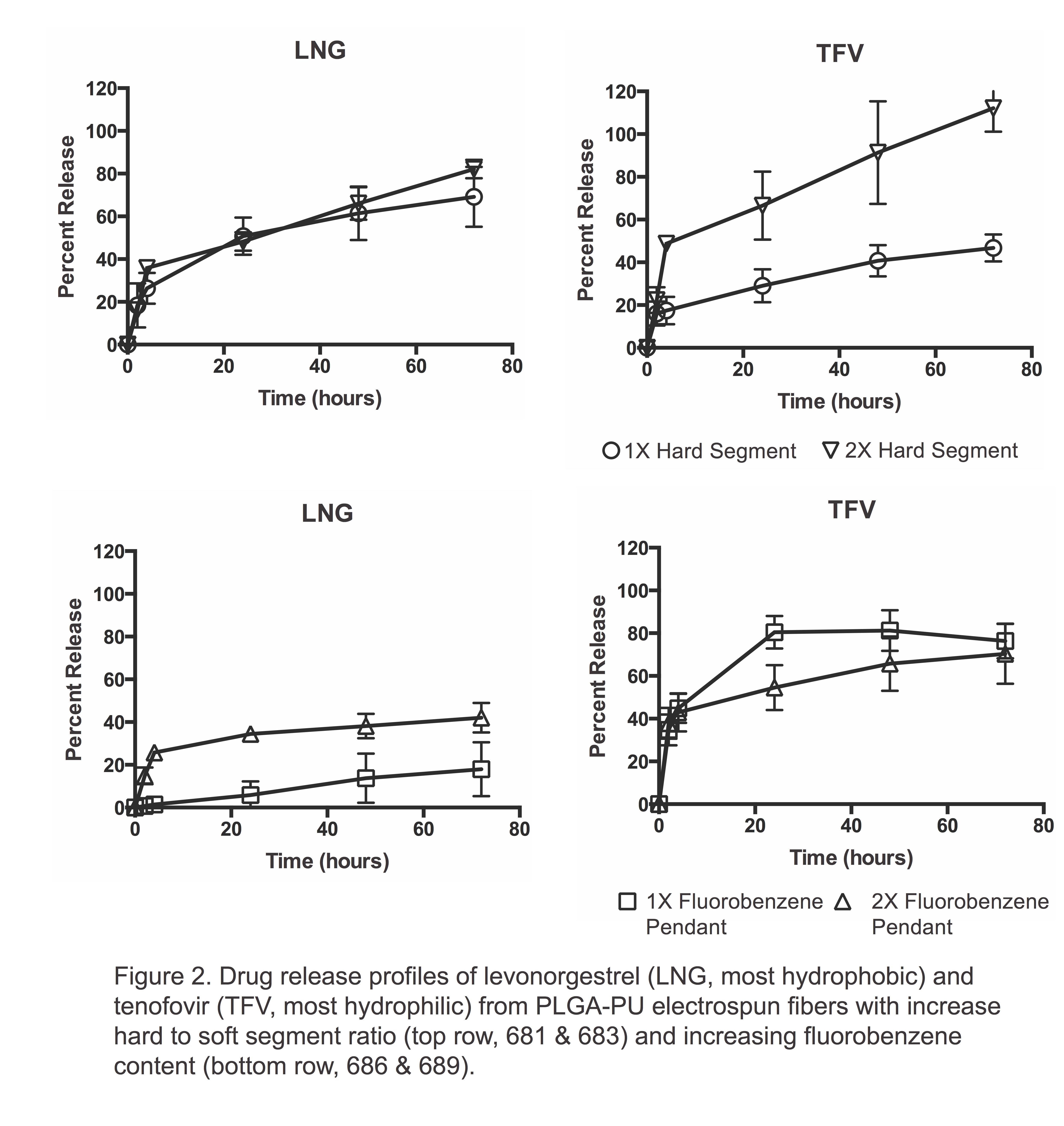
Conclusion: Increasing the hard segment or fluorobenzene content of PLGA-PUs allows for modulation of drug release of both hydrophobic and hydrophilic drugs from electrospun fibers while maintaining a biologically relevant biodegradation rate.
This work was supported by funding from NIH/NIAD and the Bill & Melinda Gates Foundation (AI112002, OPP1067729) to KAW and an NSF Graduate Research Fellowship to AKB.
References:
[1] Sill, T. J.; von Recum, H. A., Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 2008, 29 (13), 1989-2006.
[2] Agarwal, S.; Wendorff, J. H.; Greiner, A., Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603-5621.
[3] Luong-Van, E.; Grøndahl, L.; Chua, K. N.; Leong, K. W.; Nurcombe, V.; Cool, S. M., Controlled release of heparin from poly(epsilon-caprolactone) electrospun fibers. Biomaterials 2006, 27 (9), 2042-2050.
[4] Heijkants, R.; van Calck, R. V.; van Tienen, T. G., Uncatalyzed synthesis, thermal and mechanical properties of polyurethanes based on poly (ε-caprolactone) and 1, 4-butane diisocyanate with uniform hard segment. Biomaterials 2005, 26, 4219-4228.
[5] Santerre, J. P.; Woodhouse, K.; Laroche, G.; Labow, R. S., Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials 2005, 26, 7457-7470.
[6] Rockwood, D. N.; Woodhouse, K. A.; Fromstein, J. D.; Chase, D. B.; Rabolt, J. F., Characterization of biodegradable polyurethane microfibers for tissue engineering. Journal of Biomaterials Science-Polymer Edition 2007, 18 (6), 743-758.
[7] Galperin, A.; Smith, K.; Geisler, N. S.; Bryers, J. D.; Ratner, B. D., Precision-Porous PolyHEMA-Based Scaffold as an Antibiotic-Releasing Insert for a Scleral Bandage. ACS Biomaterials Science & Engineering 2015, 1 (7), 593-600.
[8] Guan, J.; Sacks, M. S.; Beckman, E. J.; Wagner, W. R., Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J. Biomed. Mater. Res. 2002, 61 (3), 493-503.