Introduction: In recent years, the introduction of non-viral gene transfer systems for treatment of inherited and acquired liver diseases has attracted a lot of attention. To mediate liver-directed gene delivery, the strategy of hepatocyte cell targeting and intracellular control of gene trafficking for designing an ideal non-viral gene delivery system are a crucial and great challenge. In order to meet this needs, we developed a novel multifunctional gene carrier, polylactitol-based gene transporter (PLT).
Materials and Methods: Poly(lactitol-co-PEI) (PLT) prepared by simple chemical processes: Lactitol diacrylate (LDA) was prepared by reaction between lactitol and acryloyl chloride (molar ratio 1:2). After preparation of LDA, PLT was synthesized with a Michael addition reaction of lactitol diacrylate and low MW BPEI (MW: 1.2 kDa) at a molar ratio of 1:1 as schematically described in Figure 1.

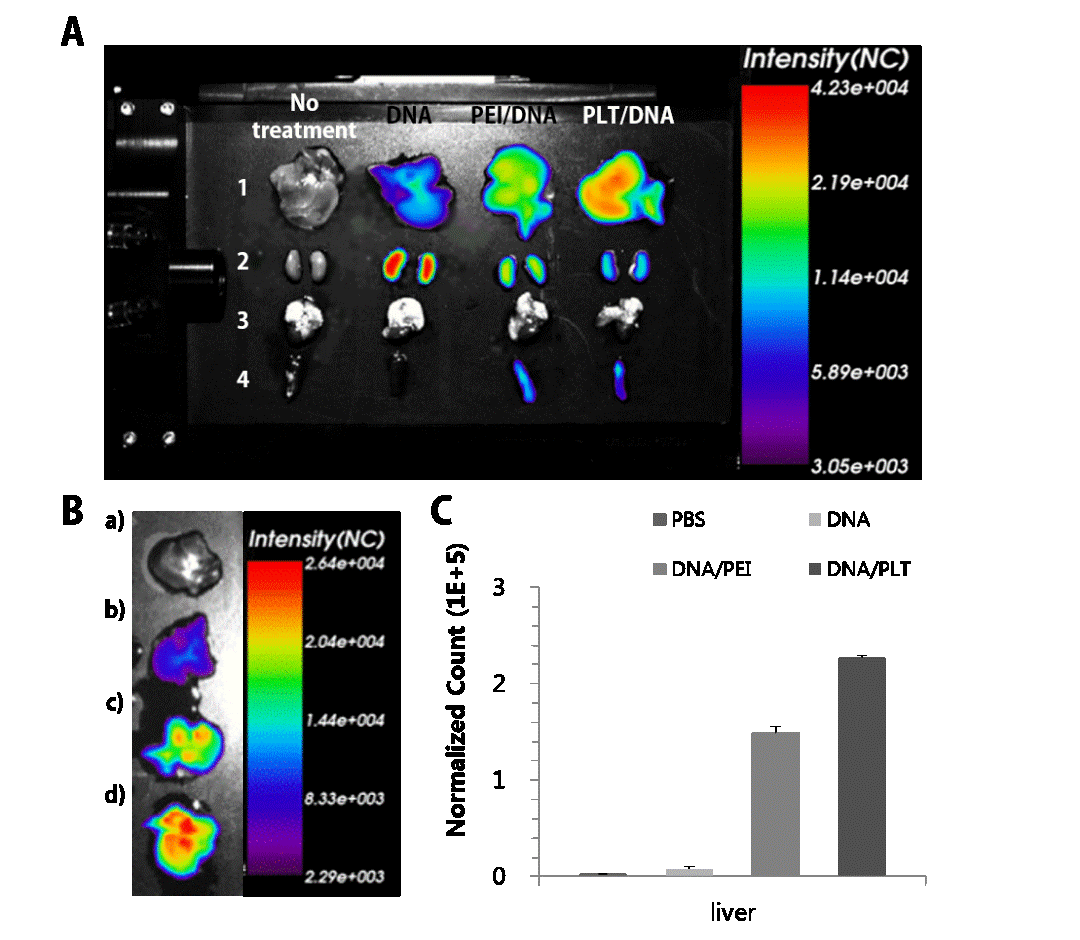
After that, we confirmed physicochemical properties, cell cytotoxicity, transfection efficiency, hepatocyte cell targeting ability, and mechanism of gene transfer of PLT/DNA complexes in vitro. Furthermore, to clarify the potential of PLT for in vivo application as a gene delivery carrier, biodistribution and liver cell targeting efficacy of PLT gene transfer system were confirmed in vivo.
Results and Discussion: The PLT reveals several excellent features: (1) reduction of synthesis step provides technical ease for design and preparation of nanocarrier by lactitol composed of sorbitol and galactose, (2) a single uniform population and ideal size for employing in biological applications, (3) significantly low cytotoxicity by adopting low MW PEI and hydrolysable ester linkages, and (4) an unprecedented good transfection efficiency by the synergistic effect of each functional backbone’s unique properties: polygalactose part has superior hepatocyte cell targeting ability in vitro, as well as in vivo (Figure 2.) and facilitates receptors-mediated endocytosis. The PEI part induces a rapid endosomal escape of genes and prevents gene degradation within the late endosome and lysosome due to its proton sponge effect. The hyperosmotic activity of polylactitol can selectively stimulate the caveolae-mediated endocytosis avoiding lysosomal degradation in similar fashion to that of polyol-based gene transporters using polyglycerol[1],[2] polysorbitol[3]-[5], polymannitol [6]-[8], and polyxylitol[9],[10] in our previous studies.
Conclusion: Polylactitol-based gene transporter as a multifunctional nanocarrier for hepatocyte cell targeting through cellular regulation was designed and successfully demonstrated to be an advanced multifunctional gene delivery system. Our study demonstrated that targeted and controlled gene delivery with a polylactitol-based gene transporter provides a new insight and concept for designing a multifunctional gene nanocarrier and a solution to solve several barriers in therapeutic applications of liver-directed non-viral gene therapy in the hepatic diseases.
The National Research Foundation of Korea, through the Oromaxillofacial Dysfunction Research Center for the Elderly (No. 2014050477) at Seoul National University in Korea
References:
[1] R. B. Arote, S. K. Hwang, M. K. Yoo, D. Jere, H. L. Jiang, Y. K. Kim, Y. J. Choi, J. W. Nah, M. H. Cho and C. S. Cho, The journal of gene medicine, 2008, 10, 1223-1235.
[2] R. B. Arote, E. S. Lee, H. L. Jiang, Y. K. Kim, Y. J. Choi, M. H. Cho and C. S. Cho, Bioconjugate chemistry, 2009, 20, 2231-2241.
[3] M. A. Islam, C. H. Yun, Y. J. Choi, J. Y. Shin, R. Arote, H. L. Jiang, S. K. Kang, J. W. Nah, I. K. Park, M. H. Cho and C. S. Cho, Biomaterials, 2011, 32, 9908-9924.
[4] M. A. Islam, J. Y. Shin, J. Firdous, T. E. Park, Y. J. Choi, M. H. Cho, C. H. Yun and C. S. Cho, Biomaterials, 2012, 33, 8868-8880.
[5] Q. P. Luu, J. Y. Shin, Y. K. Kim, M. A. Islam, S. K. Kang, M. H. Cho, Y. J. Choi and C. S. Cho, Molecular pharmaceutics, 2012, 9, 2206-2218.
[6] T. E. Park, B. Kang, Y. K. Kim, Q. Zhang, W. S. Lee, M. A. Islam, S. K. Kang, M. H. Cho, Y. J. Choi and C. S. Cho, Biomaterials, 2012, 33, 7272-7281.
[7] T. E. Park, B. Singh, H. Li, J. Y. Lee, S. K. Kang, Y. J. Choi and C. S. Cho, Biomaterials, 2015, 38, 61-71.
[8] P. Garg, S. Pandey, B. Kang, K.-T. Lim, J. Kim, M.-H. Cho, T.-E. Park, Y.-J. Choi, P.-H. Chung, C.-S. Cho and J. H. Chung, Journal of Materials Chemistry B, 2014, 2, 2666-2679.
[9] W. S. Lee, Y. K. Kim, Q. Zhang, T. E. Park, S. K. Kang, D. W. Kim, C. S. Cho and Y. J. Choi, Nanomedicine : nanotechnology, biology, and medicine, 2014, 10, 525-534.
[10] P. Garg, S. Pandey, H. Seonwoo, S. Yeom, Y. H. Choung, C. S. Cho, P. H. Choung and J. Hoon Chung, Chemical communications, 2015, 51, 3645-3648.