Introduction: The sustained delivery of both hydrophobic and hydrophilic drugs from hydrogels remains a challenge that requires the design of complex multifunctional synthetic polymers. In this work we exploit mucin glycoproteins, the gel-forming building blocks of natural mucus to deliver both hydrophobic and hydrophilic drugs. Mucins are a family of high molecular weight proteins which are densely glycosylated. Their central protein backbone contains hydrophobic and charged domains, while the mucin-associated glycans provide hydrogen bonding capabilities, high hydration, and negative charges. The biochemical versatility of mucins represent potential binding sites for certain drugs. If assembled into hydrogels, mucins could prevent drugs from freely diffusing out, leading to their sustained delivery.
Materials and Methods: We generated methacrylated mucins which assembled into a covalently crosslinked mucin hydrogel when exposed to UV light. The gel's structure was observed by scanning electron microscopy, its rheological properties measured using a parallel plate rheometer, and its degradation profile when exposed to proteases was assessed by measuring weight changes. Fluorescently labeled paclitaxel, a hydrophobic anticancer drug and polymyxin B, a positively charged hydrophilic antibiotic were chosen as model drugs. The drugs were mixed with the mucin before gel formation, and their release from the gel followed over seven days. The activity of the released drugs was tested by measuring the decrease in viability of HeLa epithelial cell when exposed to paclitaxel and the killing of E. coli bacteria by polymyxin B.
Results and Discussion: The rheological properties of the mucin gels were dominated by an elastic component, and the storage and loss moduli were maintained over four weeks when stored in buffer at 37ºC. The gel was resistant to the alpha-chymotrypsin protease, but showed some degradation in pronase. Our data show that paclitaxel, a model hydrophobic anticancer drug and polymyxin B, a positively charged hydrophilic model antibiotic drug, are retained in the gel and release linearly over more than seven days. This was in contrast with the burst release of the negatively charged poly-glutamic acid and neutral dextran molecules.
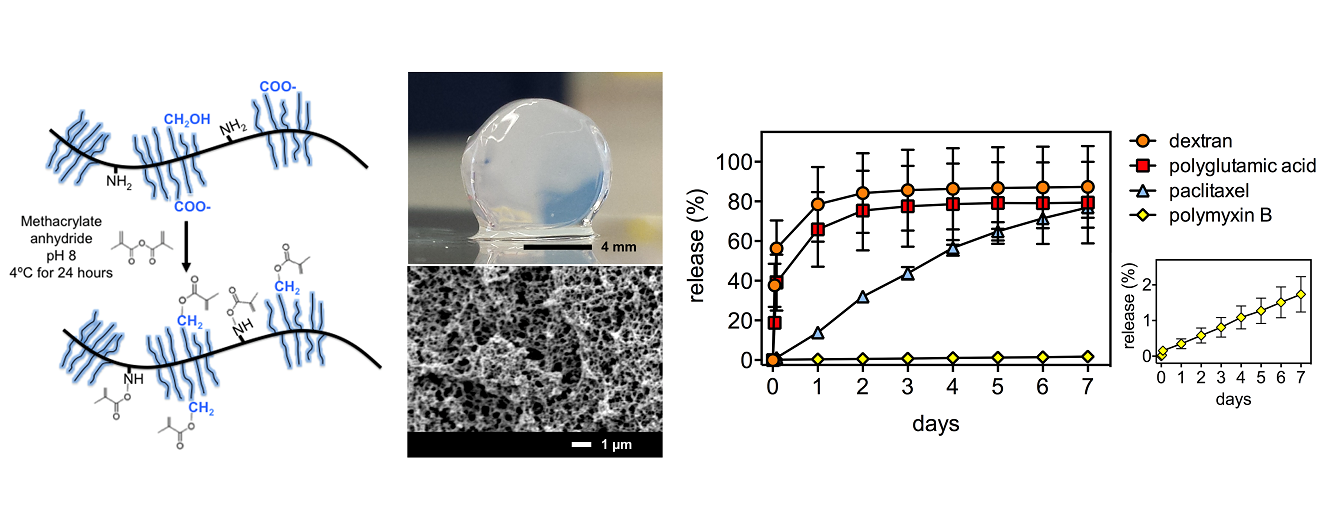
We show that after three weeks of release, sufficient amounts of active paclitaxel were present to reduce HeLa cell viability. In addition, we show that the mucin gels can sequester polymyxin B and release it in sufficient amounts to inhibit bacteria growth over a month.
Conclusions: This work suggests that mucins may have potential as a new building block for functional biomaterials that could exploit the divers set of functionalities mucins polymers naturally exhibit[1]. Amongst others, these include: interactions with drug, proteins, lipids, viruses, bacteria and immune cells, strong hydration, lubrication, and cell repellent properties.
References:
[1] Duffy, C. V., David, L. & Crouzier, T. Covalently-crosslinked mucin biopolymer hydrogels for sustained drug delivery. Acta Biomater. 20, 51–59 (2015).