Introduction: Chitosan hydrogels are able to provide local delivery of loaded therapeutic agents but their delivery can be rapid and not easily time-controllable due to, particularly, the burst effect. This leads to a loss in drug efficiency and lifetime. To overcome the consequences of burst effect, systems involving liposomes incorporated into chitosan hydrogels may appear as a promising material. The goal of this study is to develop drug loading liposomes incorporated in chitosan hydrogels able to accurately control the kinetics of drug release, avoiding the burst effect, and to model the kinetics mechanism.
Materials and Methods: Chitosan Medium Molecular Weight (C, weight average molar mass of 210,000 g.mol-1 and degree of acetylation of 25%), gelatin B (G) of bovine origin, Phospholipon-90G (phosphatidylcholine) were used. MLVs were obtained by the thin film hydration method[1] and SUVs by sonication method[2]. In order to achieve a controlled release of calcein, complex systems were prepared. As a first stage, calcein was encapsulated in liposomes. The suspension of calcein loaded liposomes was then added to the polymer solution prior to the introduction of GA as covalent crosslinking agent[3]. Then the crosslinking process continued with the introduction of ionic crosslinker (Sodium TripolyPhosphate-TPP, or sodium sulphate-S)[4].
Results and Discussion: The release of calcein was first studied according to the nature of the ionic crosslinking agent (sodium sulphate or tripolyphosphate)[4]. For the CG-S hydrogels about 80% of the loaded drug was gradually released into the supernatant over seven days. A similar behavior was observed for CG-T hydrogels but, there was a lower efficiency of calcein release 65% due to higher crosslinking density. From kinetics studies and modeling of kinetics curves we have shown that interpenetrated networks based on double-crosslinked chitosan are capable of releasing hydrophilic drugs through a multi-scale mechanism (Figure 1), characterized by four distinct phases, each characterized by a different kinetics model[5]: 1) the burst effect described by Higuchi equation; 2) the swelling of the polymer matrix, following the Korsmeyer and Peppas model; 3) the equilibrium phase - constant concentration and can be analyzed by two equations, Peppas-Sahlin and Weibull. The analysis of the equation parameters shows the preponderance of the Fickian mechanism compared to the polymer matrix relaxation.

The inclusion of liposomes allows to decrease the undesirable burst effect (Figure 2). Systems containing MLVs release higher amounts of calcein compared to systems containing SUVs, although these liposomes are more stable in the matrix and diffuse with difficulty. The explanation is the fact that MLVs transport a higher quantity of calcein, in relation with teir volume). Mathematical modeling of the experimental data confirms these observations.
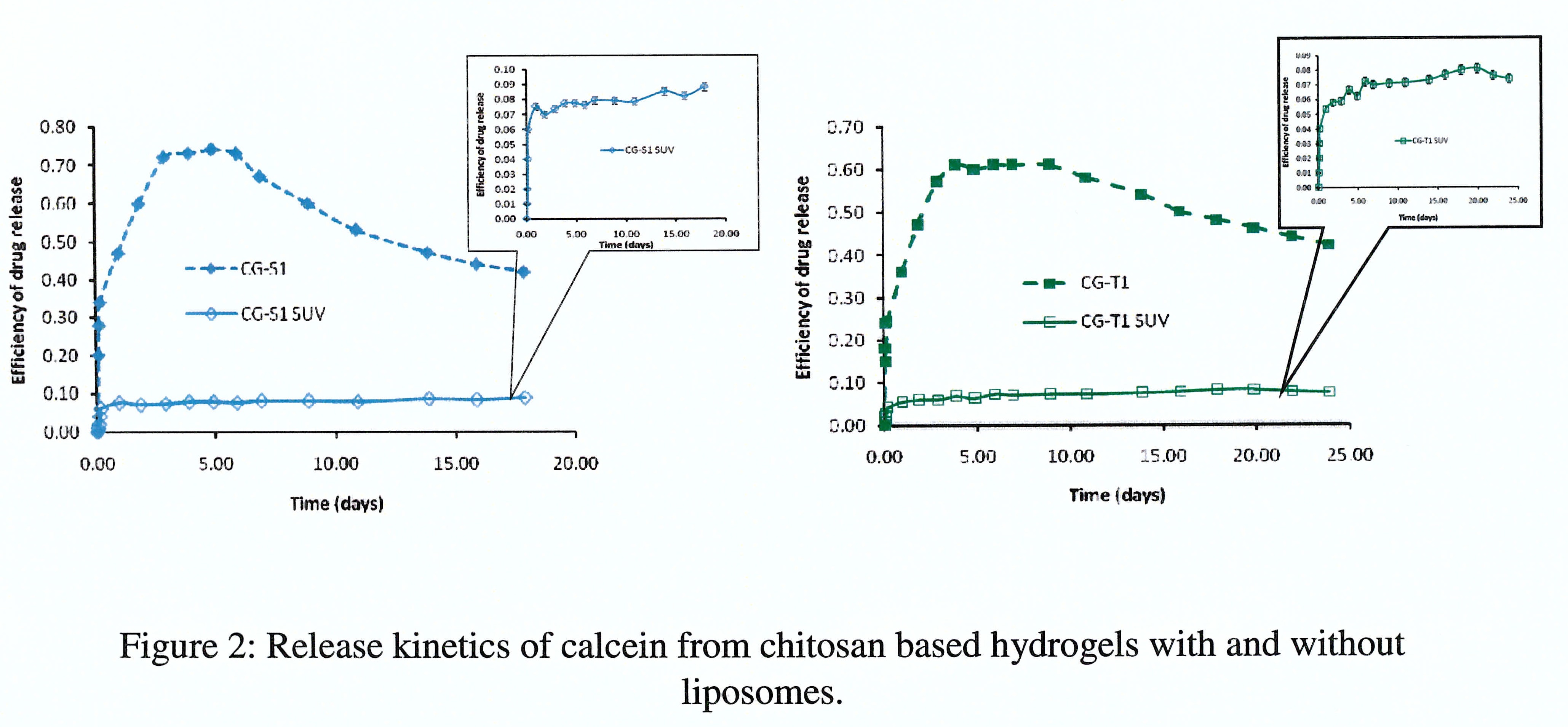
Conclusion: The use of liposomes entrapped within chitosan hydrogels allows a strong decrease of burst effect as well as a better control of release kinetics. The release kinetics was modelled and its knowledge will be a very interesting tool for, on one hand understanding the most pertinent mechanisms involved, and on another hand designing new formulations for tissue engineering, regenerative medicine and drug delivery systems.
References:
[1] Peptu C.A., Buhus G., Popa M., Perichaud A. and Costin D., Double crosslinked chitosan-gelatin particulate systems for ophthalmic applications. 2010, J. Bioact. Compat. Polym., Vol. 25, pg. 198-216.
[2] Popescu M.-T., Mourtas S., Pampalakis G., Antimisiaris S.G. and Tsitsilianis C., pH-Responsive Hydrogel/Liposome Soft Nanocomposites For Tuning Drug Release, 2011, Biomacromolecules, Vol. 12, pg. 3023–3030.
[3] Peptu C.A., Popa M. and Antimisiaris S.G., Release of Liposome-Encapsulated Calcein from Liposome Entrapping Gelatin-Carboxymethylcellulose Films: A Presentation of Different Possibilities, 2008, Journal of Nanoscience and Nanotechnology, Vol.8, pg. 1-10.
[4] Ciobanu B.C., Cadinoiu A.N., Popa M., Desbrieres J. and Peptu C.A., Modulated release from liposomes entrapped in chitosan/gelatin hydrogels. 2014, Materials Science and Engineering C, Vol. 43, pg. 383-391.
[5] Bacaita E.S., Ciobanu B.C., Popa M., Agop M. and Desbrieres J., Phases in the temporal multiscale evolution of the drug release mechanism in IPN-type chitosan based hydrogels. 2014, Phys. Chem. Chem. Phys., Vol. 16, pg. 25896-25905.