Introduction: Whole organ engineering of the liver has emerged as a potential solution for patients with end-stage liver failure. However, a major challenge of this approach is the loss of hepatic specific function after hepatocytes are removed from their native microenvironment. The identification of a soluble or solid substrate that can maintain normal hepatocyte phenotype during in-vitro culture would represent a significant advancement. Previous work has shown that hepatocytes better maintain phenotype and functionality when cultured on liver specific extracellular matrix (ECM)[1][2]. The objective of the present study was to compare the ability of porcine, human, rat, and canine liver ECM to support primary hepatocytes culture in-vitro.
Materials and Methods: Whole liver from human, porcine, canine, rat was decellularized, lyophilized, and enzymatically digested (solubilized) with pepsin. Primary rat hepatocytes (PRHs) were isolated and cultured on rat tail type I collagen coated plates for 24 hours in hepatocyte basal media. Media was then changed with the addition of 50 µg/mL of solubilized human liver ECM (Human LECM), porcine LECM, canine LECM, or rat LECM. Media alone, pepsin, solubilized urinary bladder matrix and small intestinal submucosa were included as controls. Conditioned media was collected on days 2 and 5 and tested for albumin production. Morphology images were taken on days 2, 5, and 7. Ammonia metabolism was measured on day 7.
Results and Discussion: Preliminary data shows PRHs treated with canine and porcine LECM showed normal hepatocyte morphology throughout the 7 day culture (figure 1). PRHs treated with canine and porcine LECM showed also showed enhanced albumin secretion and ammonia metabolism compared to the other treatment groups (figure 2).
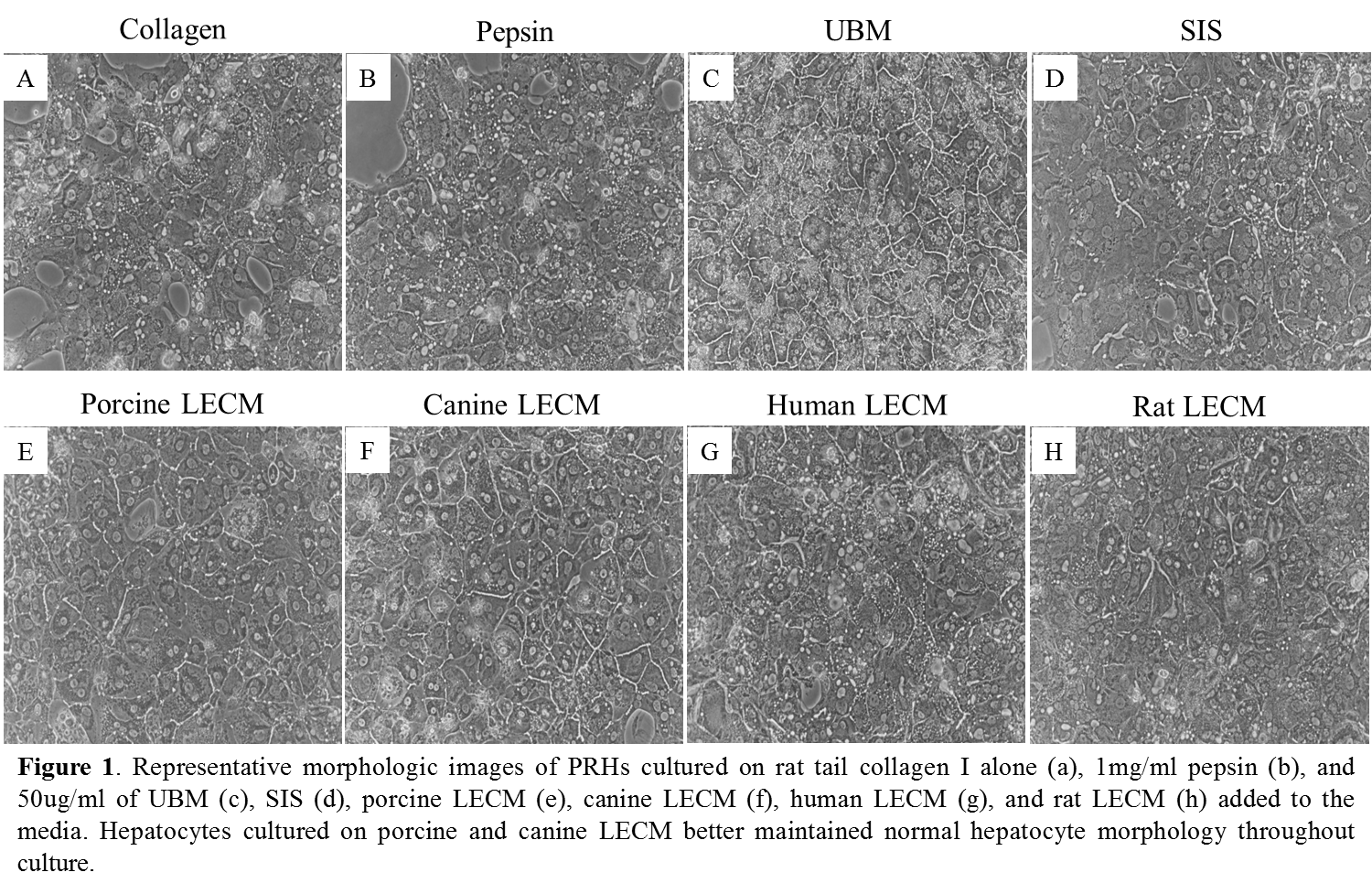
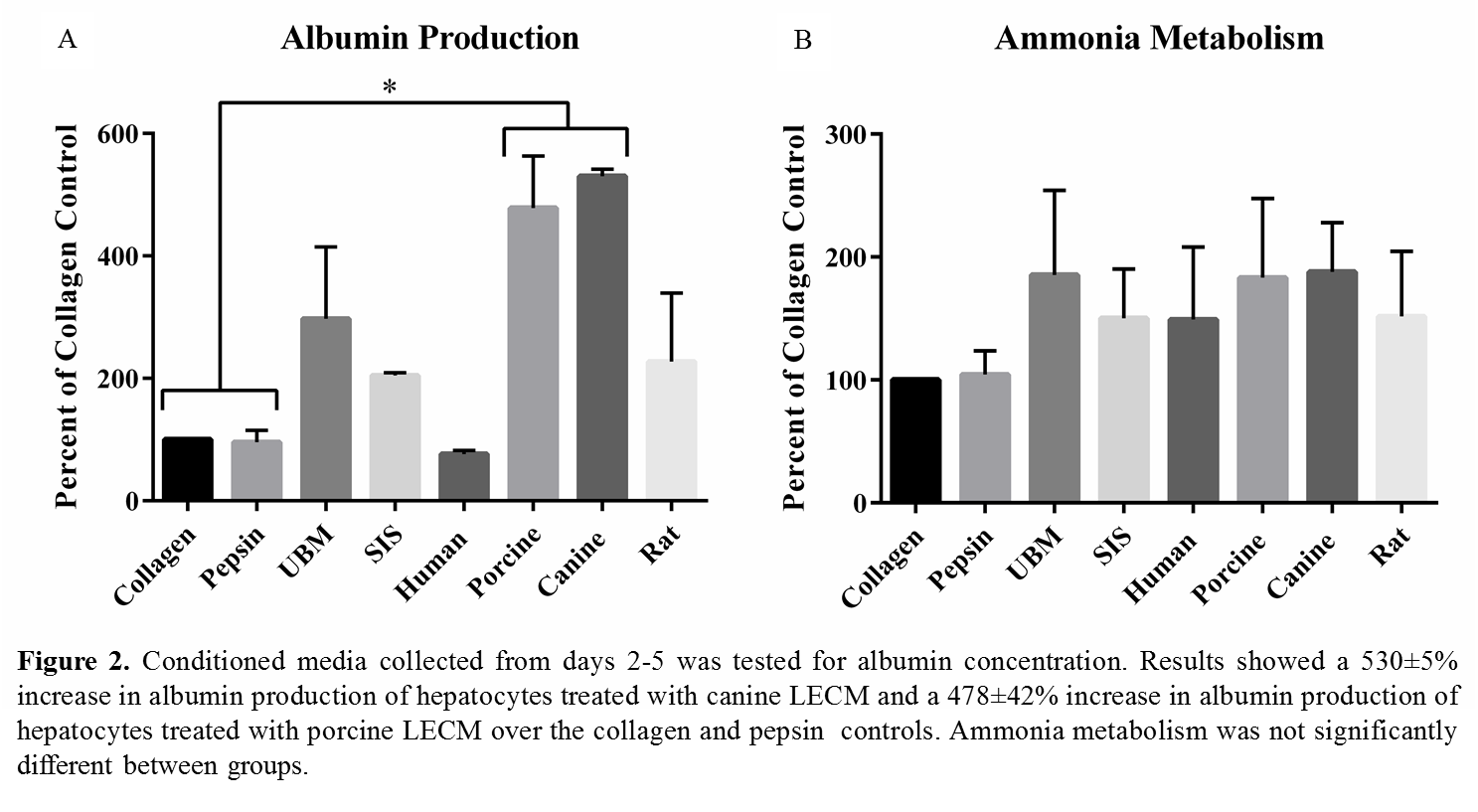
Conclusions: Solubilized ECM derived from human, porcine, canine, and rat liver was shown to support PRH function better than pepsin or rat type 1 collagen. The enhanced hepatocyte-specific functions of PRHs was seen to a greater degree with porcine and canine liver ECM, which may be due to the composition of the respective ECM. Analysis of the biochemical composition of each liver ECM is currently being conducted.
As the field of tissue engineering moves toward the replacement of complex tissues, specialized scaffolds will be needed to support multiple, functional cell phenotypes. The findings of the present study suggest that species of the ECM can affect a scaffold’s ability to support cell phenotype.
References:
[1] Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. “Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds.” Tissue Eng. 2007 Sep;13(9):2301-10. PMID: 17561801
[2] Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, Strom SC. “Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels.” Tissue Eng Part A. 2010 Mar;16(3):1075-82. doi: 10.1089/ten.TEA.2008.0587. PMID: 19845461