Photocurable gelatin hydrogels for cell encapsulation
-
1
CSIRO, Manufacturing, Australia
-
2
Wenzhou Medical University, Wenzhou Eye Hospital, China
-
3
Wenzhou Institute of Biomaterials and Engineering, China
Introduction: Tissue engineering requires the implantation of cells along with a supportive scaffold. As such, degradable support scaffolds that are amenable to minimally invasive implantation are attractive. With this in mind, we have explored the use of phototriggered thiol-ene reactions as a method to produce injectable and photocurable cell supportive scaffolds that can be cured in the presence of cells (Figure 1).
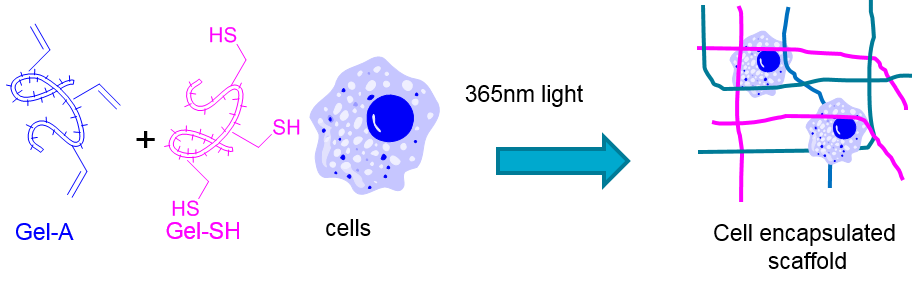
Figure 1: Conceptual diagram of in situ cured scaffold in the presence of cells
Materials and Methods: Type A Porcine skin (Bloom 100) Gelatin (Sigma Aldrich) was used for experiments. Vinyl functionalised gelatines were produced from gelatine and vinyl anhydrides (eg: acrylate and pentanoic anhydride)[1]. While thiolylated gelatin was prepared from gelatin and cysteine with EDC and NHS[1]. The products were purified by dialysis (1 kDa MWCO) and freeze dried before being reconstituted with saline (0.9 %). Light triggered thiol-ene reactions were used to crosslink the vinyl functionalised gelatins with multifunctional thiols included thiolated gelatin. The rheological properties of the precursor formulations as well as the final gels and their cure profiles were measured using photorheology. The effect of solids content, ratio of components and light intensity were investigated. The water content, optical transparency and mechanical properties and microscopic morphologies of the gels were also measured. The ability of bovine corneal endothelial cells (bCECs) and fibroblast cells (L929) to grow on the gels was investigated. The in situ curing of gels in the presence of living cells was also explored. Finally a gelatin hydrogel was cured in situ to fill corneal defects in rabbits(trephine, 3 mm diameter by 200 μm deep) to assess the corneal biocompatibility of the hydrogels.
Results and Discussion: Thiol-ene crosslinked gelatins were able to be cured within 2 min. of exposure to 365 nm light resulting in optically transparent gels with water contents about 80-85 %. The resulting soft gels possessed high viability of both L929 and bCECs (>80 %). The mechanical properties of the gels could be tuned by altering the feed ratio of the components and the light intensity used during the cure. Live/Dead staining confirmed that both bCEC and L929 retained high cell viability in culture following in situ cure of the gelatin hydrogels containing the cells (Figure 2). Moreover, laser confocal imaging indicated that the cells were evenly distributed within the hydrogel.
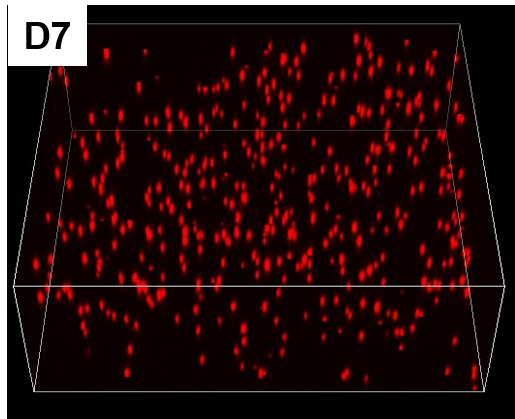
Figure 2: 3D Laser confocal fluorescent imaging of L929 cells pre stained with DilC(3) after 7 days of cell culture in a hydrogel matrix prepared from a formulation of 150 mg/ml 2:1 Gel-LA to Gel-SH + Irgacure 2959 (0.5 %) cured with 100 mW/cm2 for 1min
Corneal defects in rabbit corneas were filled with in situ cured gelatine hydrogel and the wounded cornea re-epithelialized in 3 to 7 days with minimal inflammation (Figure 3).
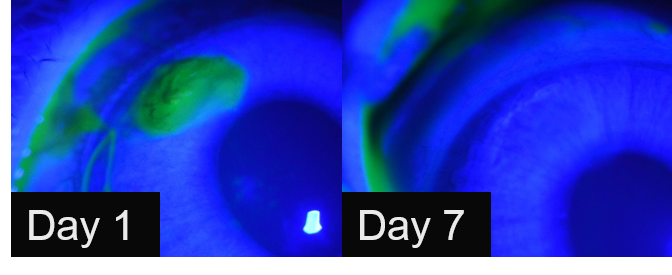
Figure 3: Slit lamp microscope images of corneal defects at day 1 (left) and 7 (right) stained with fluorescein filled with gelatin hydrogel matrix prepared from a formulation of 150 mg/ml 2:1 Gel-LA to Gel-SH + Irgacure 2959 (0.5 %) cured with 100 mW/cm2 for 1min
Conclusions: Phototriggered thiol-ene reactions are rapid and facile methods of forming crosslinked gelatin hydrogels. They resulted in transparent gels with mechanical properties suitable for soft tissue replacements. In addition, they could be cured in the presence of living cells which maintained high viability for over 7 days post crosslinking. Moreover, the in situ cured gels were able to heal rabbit corneal defects in vivo with low inflammation and rapid re-epithelialization. Therefore, the resulting materials are promising candidates for corneal tissue engineering substrates.
This study is supported by Chinese Scholarship Council, the International Scientific and Technological Cooperation Project (No. 2012DFB30020) and High-end Foreign Experts Recruitment Program (China, No.GDW20133300101).
References:
[1] Li, Lingli, et al., ‘Tissue engineering materials‘, CN104548196A.
Keywords:
Hydrogel,
Tissue Engineering,
3D scaffold,
Biodegradable material
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Biomaterials for ophthalmic applications
Citation:
Hughes
T,
Li
L,
Lu
C,
Chen
M,
Ma
H,
Wang
L,
Hao
X,
Mclean
K and
Hao
C
(2016). Photocurable gelatin hydrogels for cell encapsulation.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01497
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Timothy Hughes, CSIRO, Manufacturing, Clayton, Australia, Email1
Dr. Lingli Li, CSIRO, Manufacturing, Clayton, Australia, lingli.li@wibe.ac.cn
Dr. Conglie Lu, Wenzhou Medical University, Wenzhou Eye Hospital, Wenzhou, China, zjcilcl@163.com
Dr. Mei Chen, Wenzhou Medical University, Wenzhou Eye Hospital, Wenzhou, China, cm-8988@163.com
Dr. Huixiang Ma, Wenzhou Medical University, Wenzhou Eye Hospital, Wenzhou, China, 13777770073@163.com
Dr. Lei Wang, Wenzhou Institute of Biomaterials and Engineering, Wenzhou, China, Email2
Dr. Xiaojuan Hao, CSIRO, Manufacturing, Clayton, Australia, Xiaojuan.Hao@csiro.au
Dr. Keith Mclean, CSIRO, Manufacturing, Clayton, Australia, keith.mclean@csiro.au
Dr. Chen Hao, Wenzhou Medical University, Wenzhou Eye Hospital, Wenzhou, China, chenhao@mail.eye.ac.cn