Platelet transfusions are routinely used in the clinic to treat bleeding complications stemming from trauma, surgery, malignancy or drug related bone marrow suppression, as well as congenital defects in platelet number or function. These transfusions primarily use allogeneic natural platelet concentrates (PCs) that have limited availability, high risk of bacterial contamination, very short shelf life (~3-5 days), and several biologic side effects[1]. Due to these issues, there is a significant clinical interest in synthetic platelet substitutes that can render efficient hemostasis, leveraging and amplifying endogenous clotting mechanisms while allowing advantages of large-scale manufacture, minimum contamination risks, longer shelf-life, no need for blood-type matching and absence of biologic or pathogenic effects. To this end, we have developed a unique platelet-inspired synthetic hemostat, the SynthoPlate, which integrates platelet’s key hemostatic mechanisms of injury site-specific adhesion and aggregation by heteromultivalent surface presentation of multiple peptide types via self-assembly of lipid-peptide conjugates.
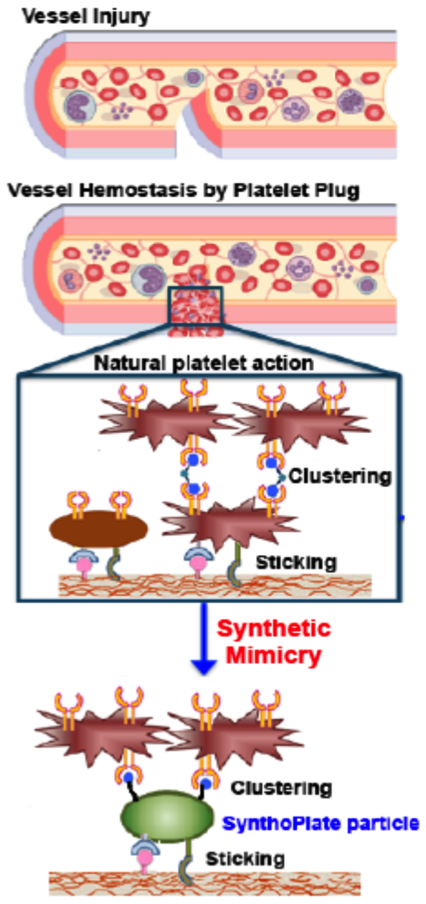
Specifically, lipids bearing vWF-binding peptides (VBP), collagen-binding peptides (CBP) and active platelet integrin GPIIb-IIIa binding fibrinogen-mimetic peptides (FMP) were self-assembled to produce ~150nm diameter SynthoPlate vesicles[2]-[4]. We first established the sterilizability and long-term stability of these particles. We then demonstrated, in vitro, that these particles render platelet-mimetic primary hemostasis mechanisms of VWF- and collagen-adhesion, and site-selective active platelet aggregation. We further demonstrated that such site-selective amplification of primary hemostatic mechanisms by SynthoPlate particles enhances secondary hemostatic output (fibrin generation), in vitro. We hypothesized that this combination of primary and secondary hemostasis enhancement by SynthoPlate will significantly reduce bleeding in both prophylactic and emergency administration scenarios, in vivo. The hypothesis was tested in appropriate tail and liver bleeding models in mice, where time for bleeding stoppage was measured. The biodistribution of the particles was also analyzed by fluorescence measurement of excised tissue homogenates. The results establish that SynthoPlate particles are capable of dose-dependently reducing bleeding time in prophylactic and emergent conditions.
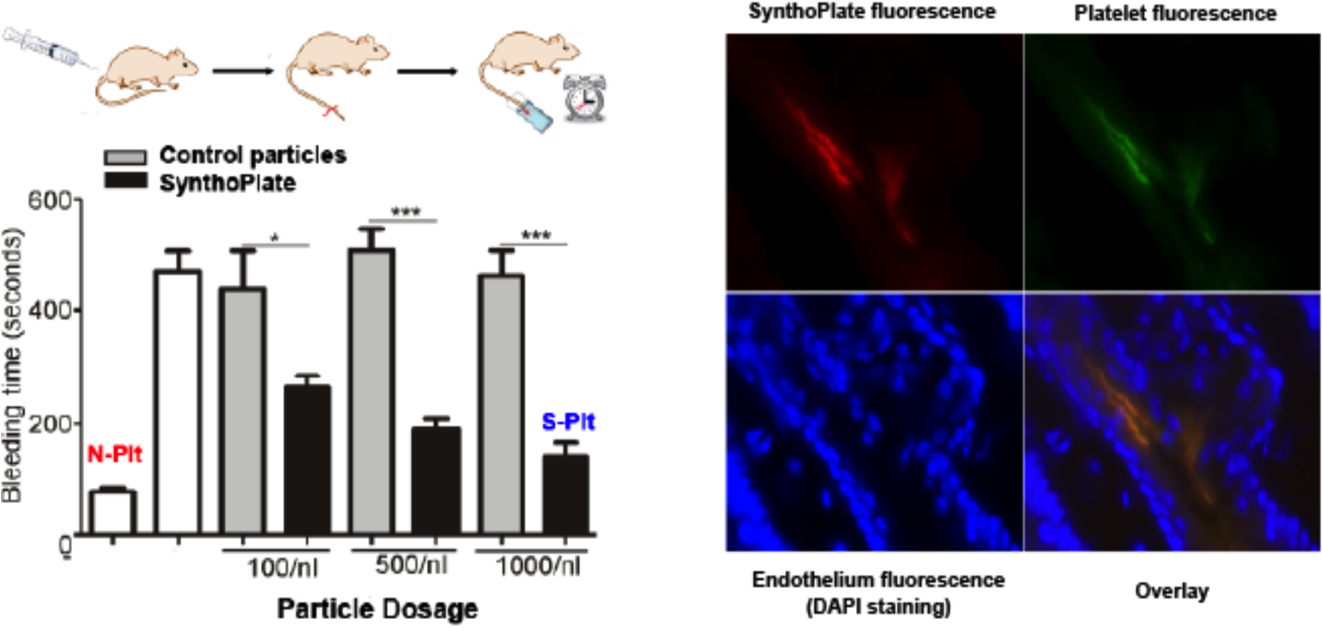
The particles are cleared mostly via the liver, spleen and kidney and have minimal accumulation in the lung. We have also demonstrated that the surface-decoration of these particles can be adapted to other particle platforms[5]. These results demonstrate the promise of the SynthoPlate technology as a platelet-mimetic intravenous synthetic hemostat. Ongoing and future studies are focused on evaluating the hemostatic efficacy of the technology in clinically motivated large animal (porcine) models of bleeding, with a vision for translation.
References:
[1] Modery-Pawlowski et al, Biomaterials 2013, 34(2): 526-541.
[2] Modery-Pawlowski et al., Biomaterials 2013, 34(12): 3031- 41.
[3] Sen Gupta and Ravikumar, 2015, US Patent No. 9107845.
[4] Sen Gupta and Modery, 2015, US Patent No. 9107963.
[5] Anselmo et al., ACS Nano 2014; 8(11): 11243-11253.