Introduction: Brain metastasis is the most devastating complication of breast cancer. Among the cancer patients, 40% are diagnosed with brain metastasis and a mean of survival of 2 months[1]. As an attempt to study the cascade that leads to brain metastasis, we have fully characterized the mouse and porcine brain to simulate the tissue mechanical properties on an in vitro model. With the addition of proteomic features, we have designed a polymer hydrogel system that mimics fundamental features of brain extracellular matrix as a platform to study breast cancer brain metastasis.
Materials and Methods: We have engineered a 3D brain extracellular matrix model using a 20K 4-arm PEG-maleimide that mimics biochemical and mechanical properties of the tissue. Mechanics of brain tissue were measured for Yorkshire pigs, six months old, and B6 albino mice, 12 weeks old, through indentation, cavitation and rheology tests. Results from these experiments can be attained in the hydrogel upon changing the polymer wt%. Biochemical features of the system were achieved by crosslinking four peptide sequences that degrade the mimic, and with 15 cell binding site peptides to facilitate cell migration and proliferation. Proteomic features for human brain were identified by immunohistochemistry and RNA-seq data[2]. The peptide concentrations in the final hydrogel will be determined using tandem mass tags technique to quantify brain tissue to be analyzed by liquid chromatography mass spectrometry.
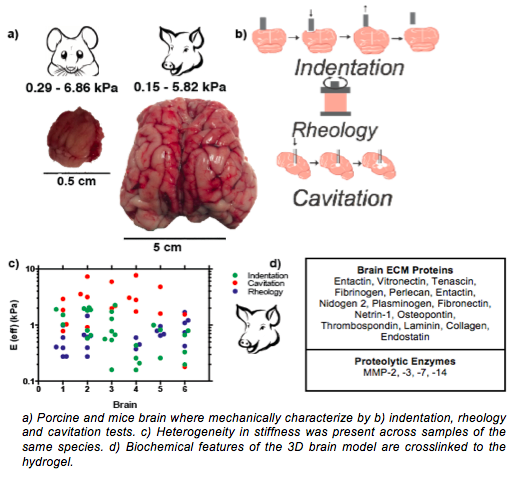
Results and Discussion: Brain tissue has an average Young’s modulus of 1.9±1.7 kPa at 25oC. Comparison of tissue stiffness across species shows no significant difference in modulus, but modulus heterogeneity was present across organ and location. Similarly, a study of stiffness variation with temperature shows that brain is independent to this effect. Stiffness present at the white matter and left hemisphere of the brain was chosen for the hydrogel given the higher breast cancer brain metastatic incidence to these locations[3],[4]. Validation of biochemical features of the hydrogel are to be tested by a competitive binding assay. It is our hypothesis that the use of healthy human cells of the brain microenvironment in the hydrogel model will better mimic the brain as found in vivo. The final model will be used to identify markers across different patient cells and a range of breast cancer cell lines to pinpoint factors that lead to an efficient breast cancer invasion and cell adhesion and proliferation.
Conclusion: The work presented shows the quantification of brain biomechanics and proteomic properties. These factors were used to design an in vitro model of the brain extracellular matrix. We can use this platform to identify markers and interactions between cancer cells and healthy cells in the brain microenvironment that lead to efficient breast cancer cell adhesion and invasion for metastasis.
NIH New Innovator Award (IDP2CA186573-01) awarded to SRP; NSF Grant (DMR-1304724) awarded to AJC
References:
[1] Fidler, I. J., Cancer Biology. 2011.
[2] Uhlén, et al, Science, 2015.
[3] Evans, A. J., et al, Clinical Oncology. 2004.
[4] Ghia, A., et al, Radiation Oncology Biology Physics. 2007.