Introduction: The success of mesenchymal stem cells (MSCs) based articular cartilage tissue engineering is limited by the presence of fibrous tissue, which is associated with the current scaffold strategy that promote cellular adhesion and spreading[1],[2]. To generate engineered cartilage with more hyaline cartilage features other than fibrocartilage, we design a scaffold based on amide bonded poly(L-glutamic acid) (PLGA) and chitosan (CS) to support in-situ adipose derived stem cells (ASCs) multicellular spheroids formation to duplicate “condensation” that takes place in vivo during limb development.
Materials and Methods: PLGA was dissolved, followed by adding N-Hydroxysuccinimide (NHS) and by1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) in turn to activate the γ-carboxyl of PLGA. After 8 h, the solution was mixed with chitosan solution to form a homogeneous and clear mixture. Then the mixture was solidified to form a hydrogel. After dialysis and lyophilization, a porous scaffold with sponge-like structure was obtained.
The third-passage ASCs were pre-labeled with fluorescent Dio dye and dropped into scaffolds. Spheroids formation in scaffolds were observed by a confocal laser microscope. To investigate chondrogenic differentiation of ASCs, cell spheroids-scaffold complex was fixed in neutral buffered formalin, embedded inembedding agent, freezing and sectioned (5 μm thick). Histology of cartilage was observed by hematoxylin and eosin (H&E), toluidine blue staining, respectively. Expression of COL II in the engineered cartilage was examined by immunohistochemical staining.
ASCs spheroids scaffold constructs after being chondrogenic induced in vitro for 2 weeks were implanted to repair articular cartilage defects at non-weight bearing area of femur trochlea on the femoropatellar groove of the knee joints.
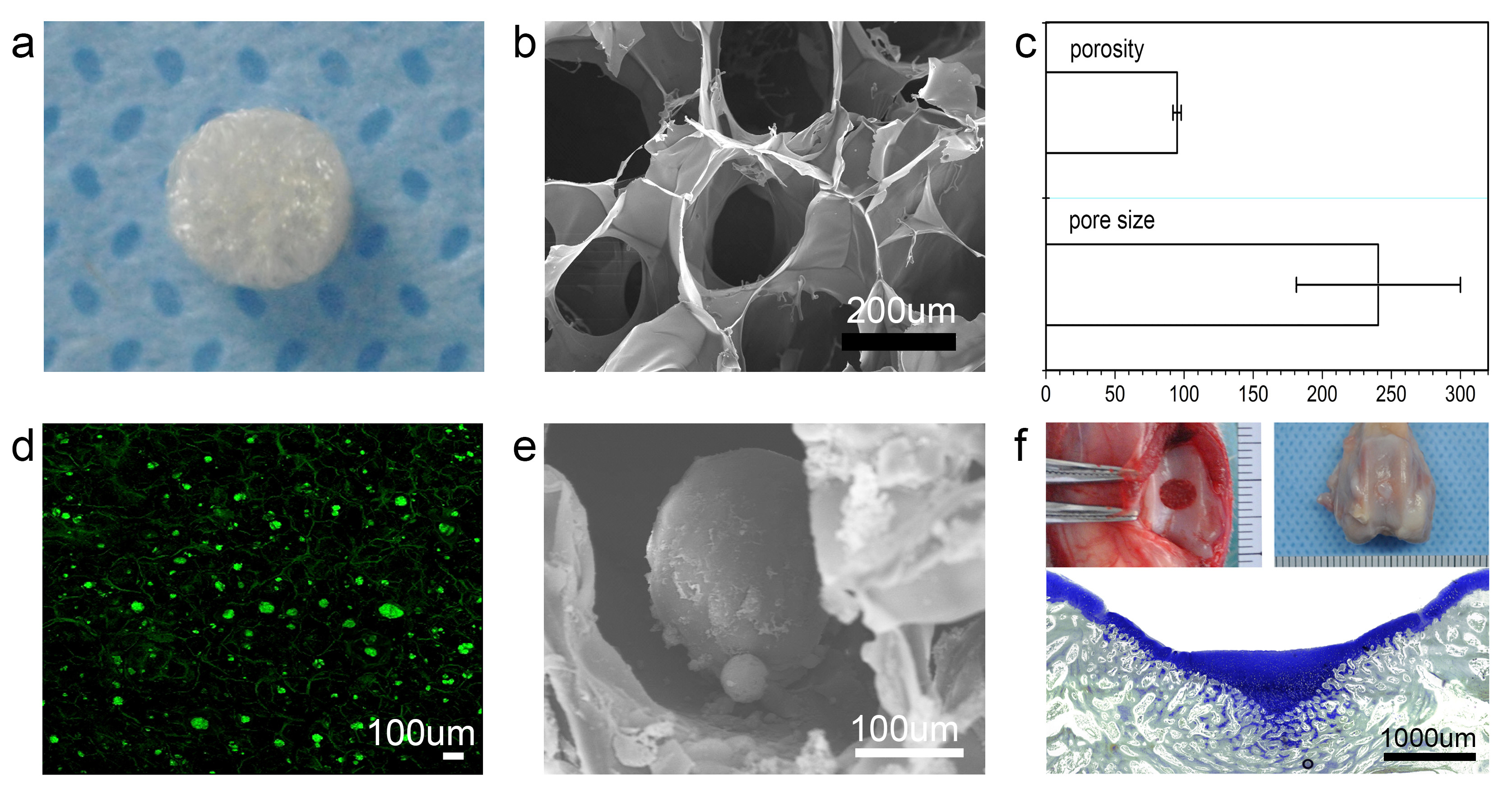
Result and Discussion: PLGA/CS sponge-like porous scaffold possessed a pore diameter of 180-300 µm when froze at -80 oC. In addition, smaller pores with the diameter of 40-60 µm were observed on the micro-pore surfaces, taking responsibility for interconnectivity (figure a,b,c).
After 4 h post-seeding, ASCs were found to disperse in pores of scaffold with rounded profile. When it came to 12 h, the appearance of numerous multicellular aggregates was found. Then, the aggregates formed spheroids with diameter of 80-110 µm (figure d,e). In vitro chondrogenic differentiation results showed that the scaffold carrying ASCs aggregates exhibited more effective chondrogenesis but limited fibrous matrix deposition.
Then, we evaluated the enhanced hyaline cartilage regeneration with implantation of ASC spheroids/scaffold in vivo. It was found that at 6 weeks post-implantation, the regenerated tissues exhibited well organized cells in columns and clusters. Cellular volume and cartilage lacuna structure were similar to normal cartilage (figure f).
Conclusion: The present strategy could induce in-situ ASC spheroids formation, thus promote intercellular interaction while reduce fibrous matrix production, showing advanced application in hyaline cartilage regeneration.
National Natural Science Foundation of China (Nos. 51173101, 51373094, 51503119); Science and Technology Commission of Shanghai Municipality (No. 15JC1490400)
References:
[1] Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science 2012;338:917-21.
[2] Nuernberger S, Cyranc N, Albrechta C, Redlb H, Vécseia V, Marlovitsa S. The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation grafts. Biomaterials 2011;32:1032-40.