Introduction: Self-assembled gel nanoparticles (also called nanogels) made of hydrophobically modified biopolymers are particularly attractive for drug delivery. Their size can be easily varied, their interior network comprising hydrophobic domains can be used to incorporate poorly water-soluble drugs and their hydrophilic shell can be exploited to control their biological fate and targeting ability. In this regard, several studies have focused on the use of hyaluronic acid (HA) for the design of anti-cancer drug carriers due to its interesting biological properties. Indeed, HA is a natural glycosaminoglycan specifically recognized by the CD44 receptor that is overexpressed by several cancer cells. In the continuing search for realizing the potential of HA-based nanoparticles, we proposed to develop new HA-based nanogels resulting from the temperature-triggered assembly of tailor-made HA-copolymer conjugates.
Experimental Methods: The HA-copolymer conjugates were synthesized using thiol-ene coupling reactions[1],[2]. The thermosensitive copolymers containing near infrared/UV light-sensitive monomers or crosslinkable monomers were synthesized using the reversible addition-fragmentation chain transfer polymerization method. The nanogels were characterized regarding physico-chemical (microscopies, dynamic light scattering) and biological (cytotoxicity and cellular uptake) properties.
Results and Discussion: We previously demonstrated that the grafting of a thermoresponsive copolymer, namely (poly(diethyleneglycol methacrylate-co-oligoethyleneglycol methacrylate) (poly(DEGMA-co-OEGMA)), onto HA allowed temperature-triggered assembly of HA into nanogels (Figure 1)[1],[3]. These nanoparticles possess many interesting features for drug delivery, like: facile formation, tunable size, easy loading of hydrophobic molecules, biodegradability of HA, selectivity for cells expressing the CD44 receptor.
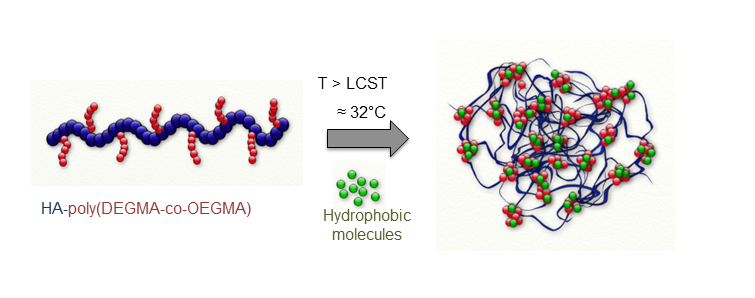
Figure 1. Formation of HA-based nanogels from thermosensitive HA-copolymer conjugates
Considering the promising properties of these self-assembled particles, we decided to focus on the design of more sophisticated nanogels by combining the advantages of the biological properties of HA and the versatile properties of copolymers. Our goal was to prepare new families of nanogels with tunable stability. Therefore, we explored original strategies for chemically crosslinking nanogels in order to increase their stability for in vivo applications. We also proposed to develop HA-based nanogels able to release their cargo on-demand by grafting new copolymers combining thermo- and light-sensitivity on HA. To this end, new thermosensitive copolymers containing functional monomers were synthesized and characterized. They were then successfully grafted on HA by thiol-ene chemistry. The resulting HA-copolymer conjugates gave rise to nanogels with sizes depending on the degree of substitution and the molar mass of HA.
Conclusion: Original chemistry approaches were developed to prepare hyaluronic acid-based nanogels with tunable stability. By varying synthesis parameters, their stability in physiological conditions could be modified depending on the pH, temperature or light (near infrared or UV) conditions. These peculiar features, in addition to the biocompatibility of the HA-copolymer conjugates, make these polysaccharide nanogels promising candidates for therapeutic delivery.
The authors would like to thank the “Agence Nationale pour la Recherche” (2014 ANR grants program: ANR-14-CE08-0014) providing financial support to this project.; MR gratefully acknowledges the MESR and the E.C. for her thesis grant in CERMAV.
References:
[1] J. Jing, D. Alaimo, E. De Vlieghere, C. Jérôme, O. De Wever, B. G. De Geest and R. Auzély-Velty, J. Mater. Chem. B (2013), 1, 3883-3887
[2] J. Mergy, A. Fournier, E. Hachet and R. Auzély-Velty, J. Polym. Sci. A Polym. Chem. (2012), 50, 4019-4028.
[3] T. F. Stefanello, A. Szarpak-Jankowska, F. Appaix, B. Louage, L. Hamard, B. G. De Geest, B. van der Sanden, C. V. Nakamura and R. Auzély-Velty, Acta Biomater. (2014), 10, 4750-4758.