Using 3D bioprinting technologies we have developed a method to embed tumor cells within a cross-linked alginate matrix and form 3D cell-laden matrices with control over the physical, chemical, and biological conditions thus mimicking the in vivo tumor microenvironment. These 3D hydrogel models allow cells to grow, proliferate and achieve higher viability than standard 2D systems while more accurately simulating physiological conditions[1],[2].
A solution of 2.66% (w/v) sodium alginate was stirred for 3 h at RT before 0.23% (w/v) CaCl2 solution was added. MDA-MB-231 and IRM-90 labeled with GFP and RFP, respectively were cultured at 37°C and 5% of CO2. Each cell type was independently mixed into alginate solutions at 1.5x106 cells/mL. The resulting cell-alginate suspensions were used to generate 3D lattice arrays using a BioScaffolder 3.1 extrusion bioprinter with a G27 dispenser tip (Fig. 1). Rheology was performed using an MCR 301 rheometer with parallel plate geometry (PP25). Mechanical Indentation experiments were performed to verify material stiffness. Scanning electron microscopy (SEM) and confocal microscopy were used to analyze the morphology of the scaffold and the presence and localization of the cells embedded within the alginate matrix.
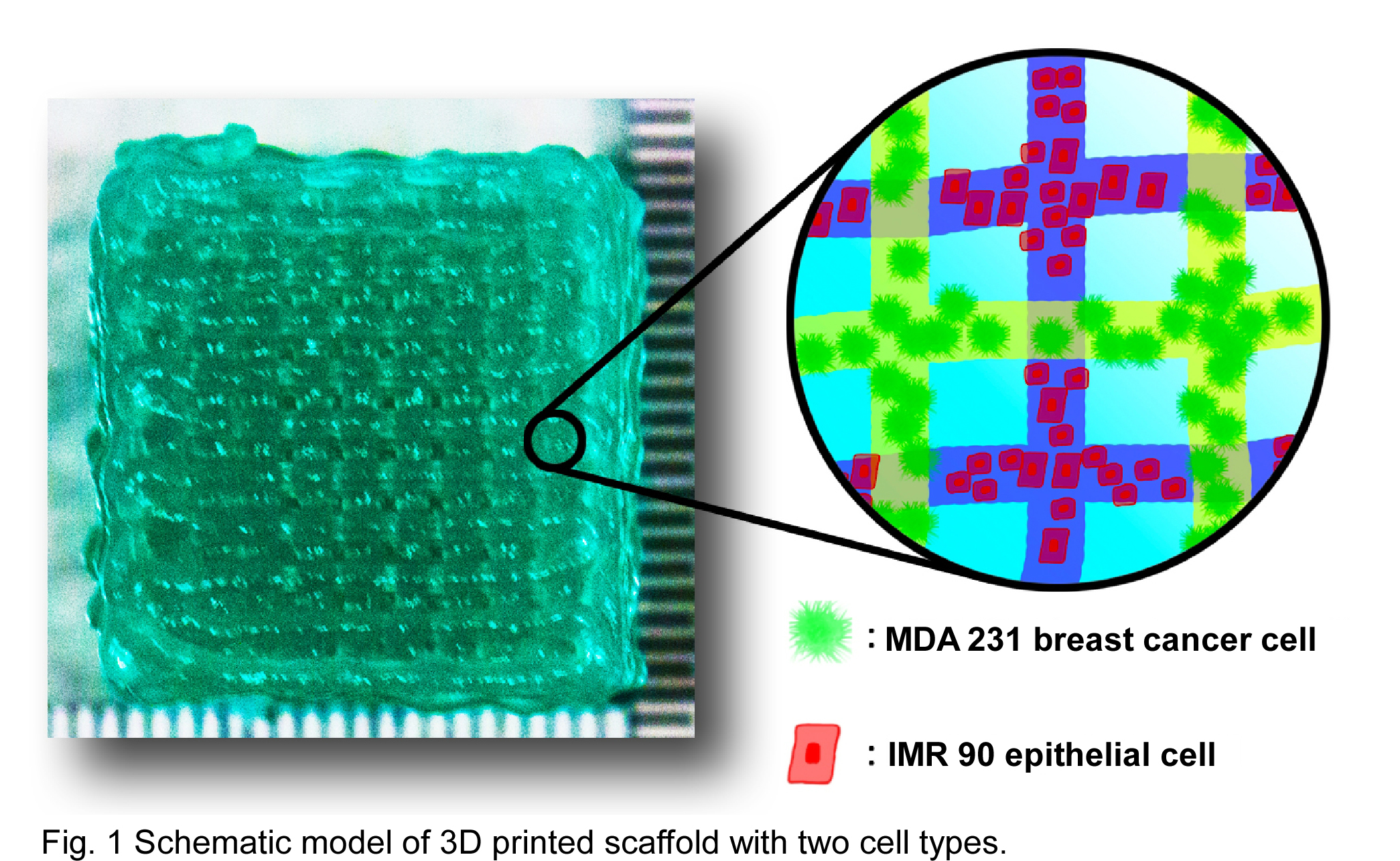
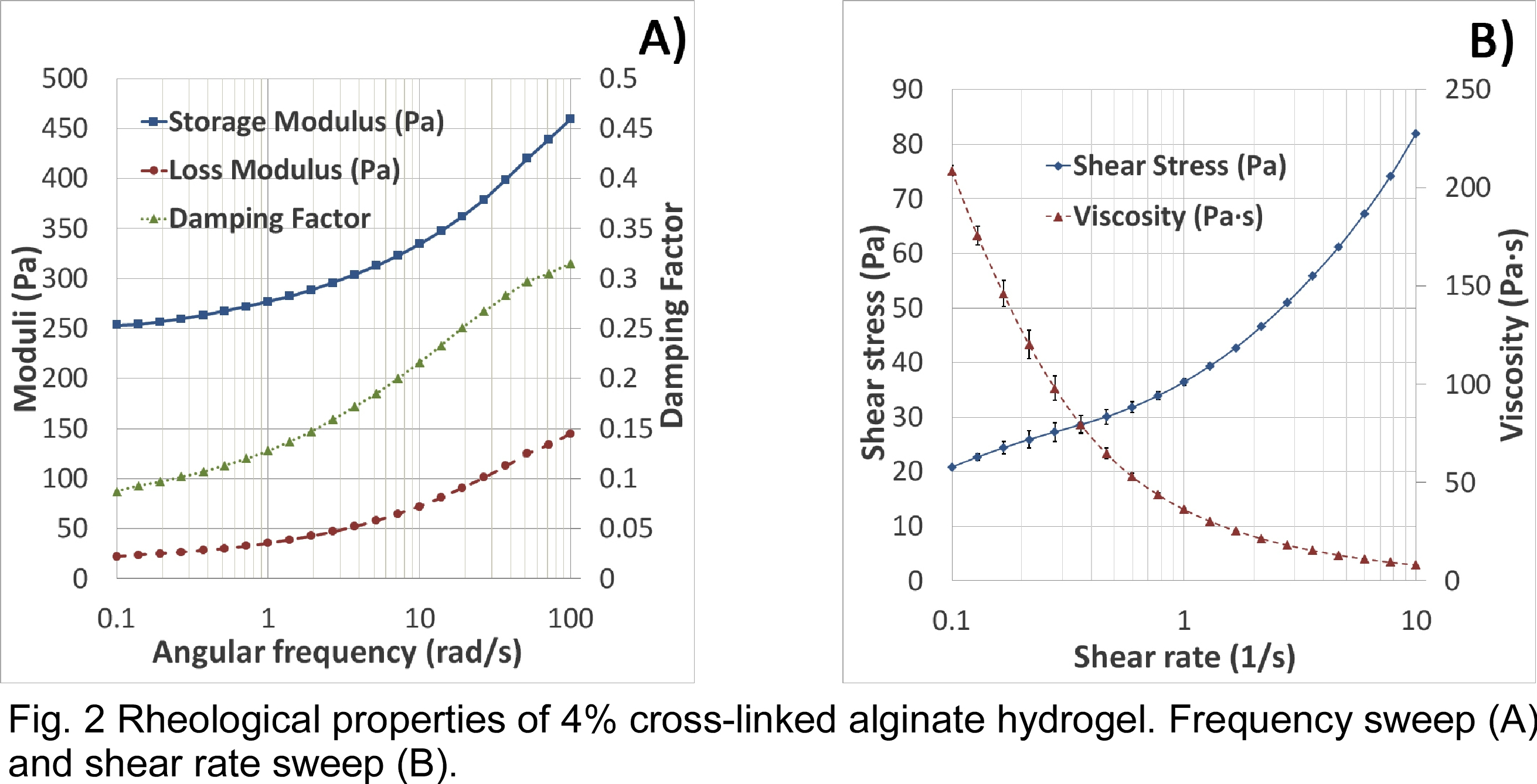
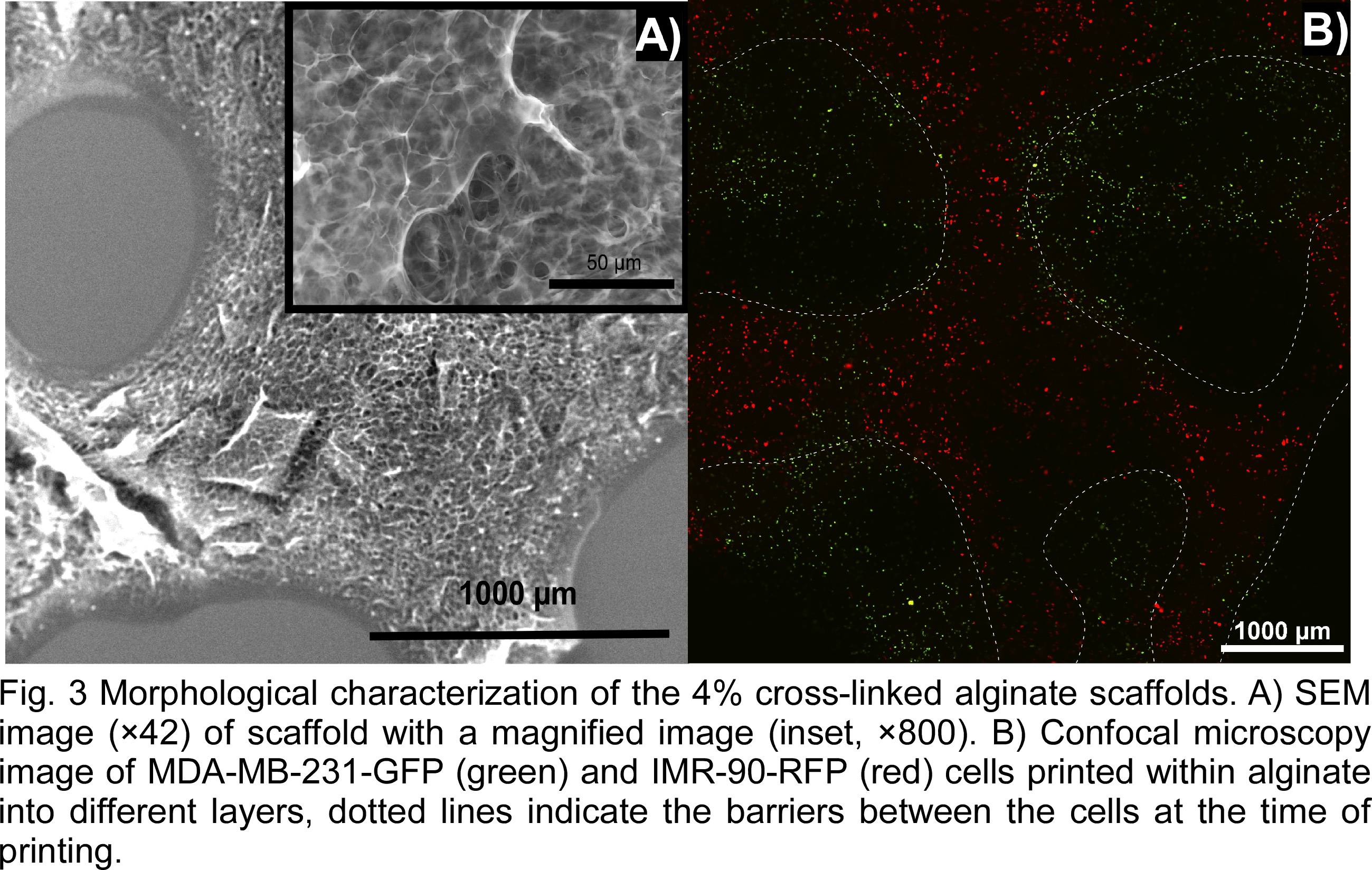
The mechanical properties of the hydrogel were measured in an effort to tune them to match those of the tumor microenvironment. The moduli of the material was measured by rheometry using a frequency sweep at 2% strain (Fig. 2 A). Storage modulus increased from 250 Pa to 460 Pa during the sweep and loss modulus increased from 25 Pa to 150 Pa. Shear rate sweep indicated that the viscosity of the alginate decreases under increasing shear rate (Fig. 2 B), indicating the shear thinning properties of the material. Indentation shows an average stiffness of 342 N/mm, which provides enough strength to avoid collapse of the 3D structure. The morphology of the matrix was observed at multiple scales using optical and electron microscopy to determine porosity and print fidelity. SEM imaging shows a high degree of micro and nanoscale porosity (Fig. 3 A), which could lead to enhanced cell growth due to increased surface area and greater exchange rates of essential nutrients and gases. Confocal imaging confirms that the cells are embedded within the alginate in a multilayer 3D matrix with control over their initial placement relative to each cell type. The two cell types were each extruded by independent cartridges creating interlaced strands (delimited by the dotted line, Fig. 3 B). This 3D model could achieve high viability and well-spread cells creating a toll to study intracellular signaling pathways within tumors. 3D bioprinting can be used to create 3D heterogeneous disease models with high-throughput, low cost, and high reproducibility as a more realistic alternative to traditional cell culture and small animal tumor models.
Volodymyr Kriuchkov; Allen Ehrlicher; Hossein khadivi heris; Adele Khavari; China Scholarship Council (CSC); McGill Engineering Doctoral Award (MEDA); Townshend-Lamarre Family Foundation; Innovative Research Award
References:
[1] S. Hong, D. Sycks, H. F. Chan, S. Lin, G. P. Lopez, F. Guilak, K. W. Leong and X. Zhao, "3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures", Adv Mater. 27 (27), 4035-40 (2015).
[2] S. Knowlton, S. Onal, C. H. Yu, J. J. Zhao and S. Tasoglu, "Bioprinting for cancer research", Trends Biotechnol(2015).