Introduction: We have developed a novel pNIPAM-DMAc-Laponite® hydrogel loaded with hydroxyapatite nanoparticles (HAPna), which can be maintained as a liquid ex-vivo and be injected into the affected bone tissue site where body temperature triggers in situ gelation. The ability of the hydrogel to induce osteogenic differentiation of mesenchymal stem cells (MSCs) in vitro, without the use of additional osteogenic inducing factors and the ability to promote bone density in vivo was investigated.
Experimental Methods: pNIPAM-DMAc-Laponite® hydrogels were synthesised and 0.5 or 1.0mg/mL HAPna were added post polymerisation. hMSCs were suspended in the liquid hydrogel, solidified and cultured for up to 6 weeks. Scanning electron microscopy (SEM) and dynamic mechanical analysis (DMA) were used to determine structure and mechanical properties. Cell viability and differentiation was determined using Alamar Blue assay, histological staining and protein expression for osteogenic markers. Following initial in vivo safety assessment using a sub cutaneous implantation. The ability of pNIPAM-DMAc-Laponite® hydrogel systems to augment bone regeneration was assessed in vivo; male wister rats were assigned to one of four experimental groups: control, acellular with HAPna, rat MSC with HAPna and rat MSC without HAPna. A single bur hole was created in the mid-shaft of the femur and filled with liquid hydrogel or left void to serve as a control. Following 4 weeks, the defect site and organs were extracted for histological examination and micro-computed tomography (Micro-CT) performed to assess bone formation, integration and potential inflammatory response.
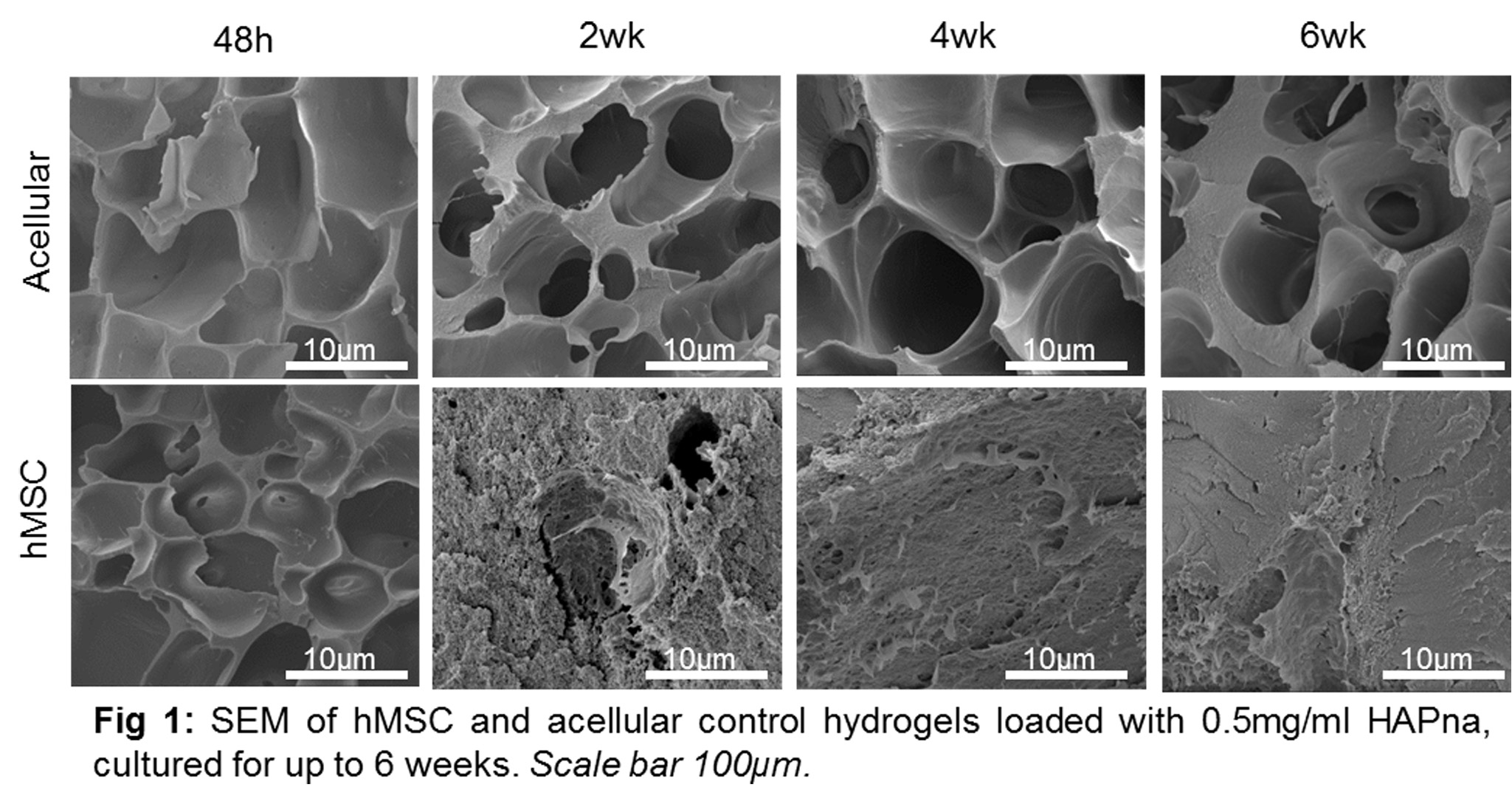
Results and Discussion: Viability of MSCs was maintained within hydrogel constructs containing 0.5mg/mL HAP-NPa, whilst some cytotoxicity was seen in 1.0mg/mL HAPna. SEM analysis demonstrated a porous structure following 48h which was rapidly filled via matrix deposition in hydrogels containing cells (Fig 1).
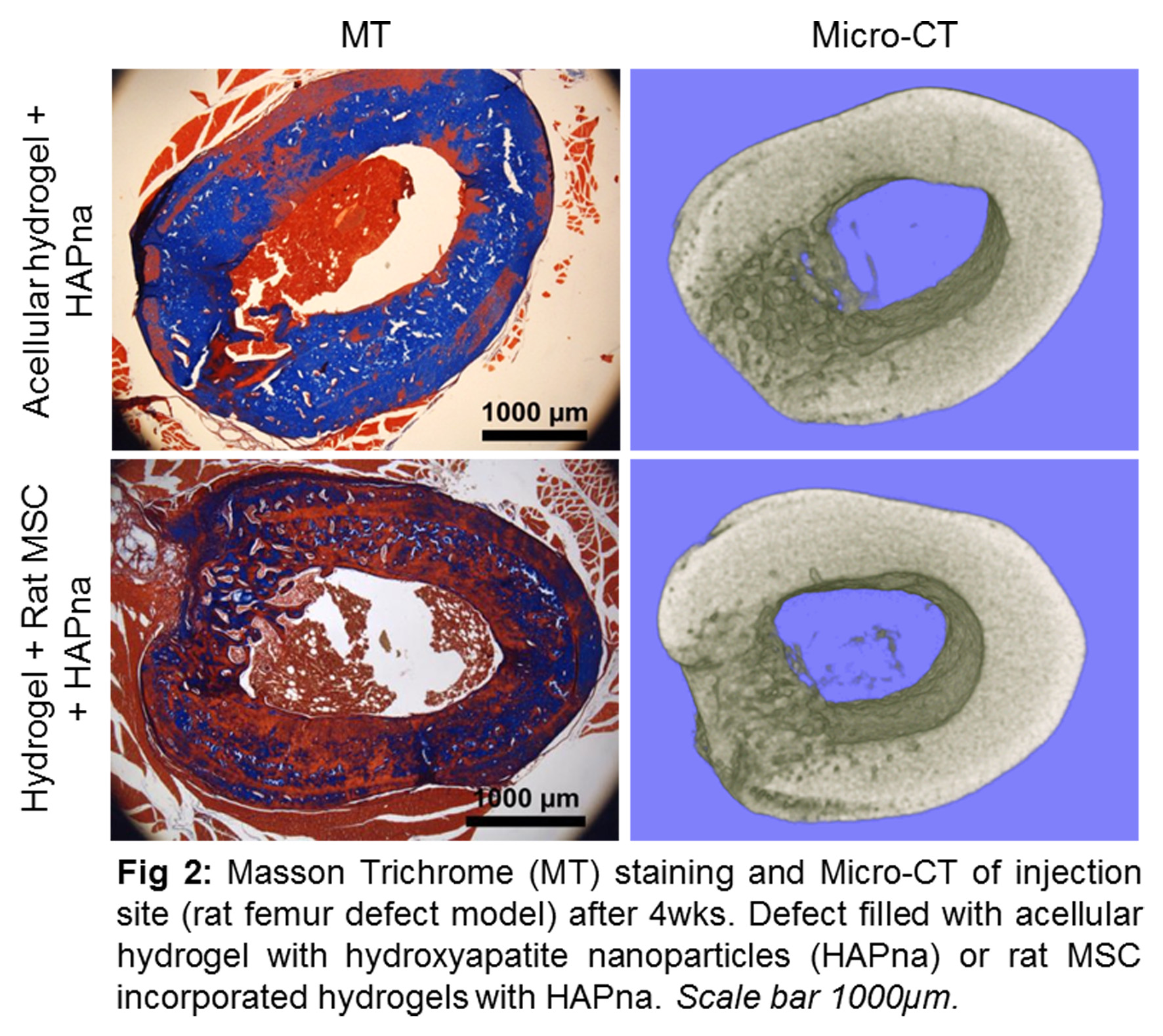
An increase in storage modulus (G’) was seen in hydrogels containing cells, with highest moduli seen in hydrogels containing 0.5mg/ml HAPna. Bone differentiation markers and collagen deposition was induced following 2 weeks with mineralised matrix observed particularly following 6 weeks. In vivo subcutaneous testing for 6 weeks demonstrated no inflammatory reaction, organ toxicity or systemic toxicity. Efficacy testing in vivo demonstrated integration of the hydrogel with surrounding bone tissue without the need for delivered MSCs, native cell infiltration was seen and excellent bone formation in hydrogels containing hydroxyapatite (Fig. 2). No signs of inflammatory reaction or organ toxicity were observed.
Conclusion: The thermally triggered hydrogel system described here was sufficient without the need of additional growth factors or osteogenic media to induce osteogenic differentiation in vitro and in vivo, such a system has great potential to reduce treatment costs and simplify the treatment strategy for orthopaedic repair and regeneration.
Funded by EPSRC (EP/H000275/1, EP/I016473/1)