Introduction: The low modulus of a load-bearing orthopaedic implant material together with its contiguous interface with the surrounding bone tissue is key to ensure a uniform load distribution and stress transfer from the implant to the bone, thus minimising stress shielding and aseptic loosening. Surface engineering of endosseous Ti implants at the nano-/micro-scale has been shown to enhance bone formation, and encourage rapid osteointegration and biomechanical stability[1]-[3]. Plasma Electrolytic Oxidation (PEO) is currently applied to dental implants made up of commercially pure α-Ti to produce bioactive TiO2-based coatings, e.g. TicerⓇ (ZL Microdent)[4], TiUniteⓇ (Nobel Biocare)[5] and BioSpark™ (Keystone Dental)[6]. Compared to commercial α- and (α+β)-type Ti alloys, β-type Ti alloys have a modulus closer to that of natural bone, and thus, potentially offer a lower stress shielding effect[7]. Also, among the three polymorphs of TiO2 (anatase, rutile and brookite), anatase has been shown to enable rapid precipitation of HA in SBF[8]; hence, it is desirable to maximize the surface anatase content. This study explores the possibility of extending the application of PEO to low-modulus β-type Ti-based alloys, while maintaining similar cellular activity as commercial implant materials.
Materials and Methods: PEO was conducted in a 10 kW 50 Hz Keronite™ rig using the current density of 20 A·dm-2 and 0.05 M Na3PO4 electrolyte. The electrolyte temperature was kept at 20∓2°C. Coatings were produced on α-Ti, (α+β)-Ti6Al4V, near β-Ti13Nb13Zr and β-Ti45Nb after 2, 5 and 30 min. The physicochemical properties of the coatings were studied using TEM, SEM, EDX, XRD, Raman, XPS, GD-OES, DSA, BET and white light interferometry. Cell response was evaluated in vitro using foetal human osteoblasts by assessing cell attachment and distribution (immunofluorescence), metabolic activity (alamarBlue), matrix formation (immunoblotting) and mineralization (OsteoImage™).
Results and Discussion: All materials showed the formation of anatase-rich, rough TiO2-based coatings exhibiting a multidimensional porous structure⏤ideal for cell-coating interdigitation. PEO coating physicochemical variation was governed by the formation of microplasma discharges, which were responsible for the simultaneous outgrowth of the oxide film containing additional alloying elements (Al, V, Nb and Zr), and deposition of the electrolyte ions (Na and P). Two mechanisms were proposed, by which the amorphous oxide was produced constantly during the PEO process, rendering the coatings anatase-rich, Fig. 1. The formation of more powerful sparks at longer dwell times altered the macro-scale morphology of the coatings, and raised the local surface phosphorus content through coating displacement and buildup. Coating roughness, thickness and substrate mass also increased with dwell time for all materials. In vitro biological evaluation of the coatings showed similar osteoblast metabolic activity, proliferation and differentiation on all materials. The anatase-rich content of the coatings did not influence the cell response, as the top surface in contact with the cells appeared to be largely amorphous, Fig. 1. Increased cell mineralization was observed at longer dwell times, which may be attributed to enhanced surface phosphorus content, Fig. 2. Cell interdigitation into the porous coatings was also observed, which could presage osteointegration in vivo, and future in vivo experiments are required to determine applicability to load-bearing orthopaedic implants, Fig. 2.
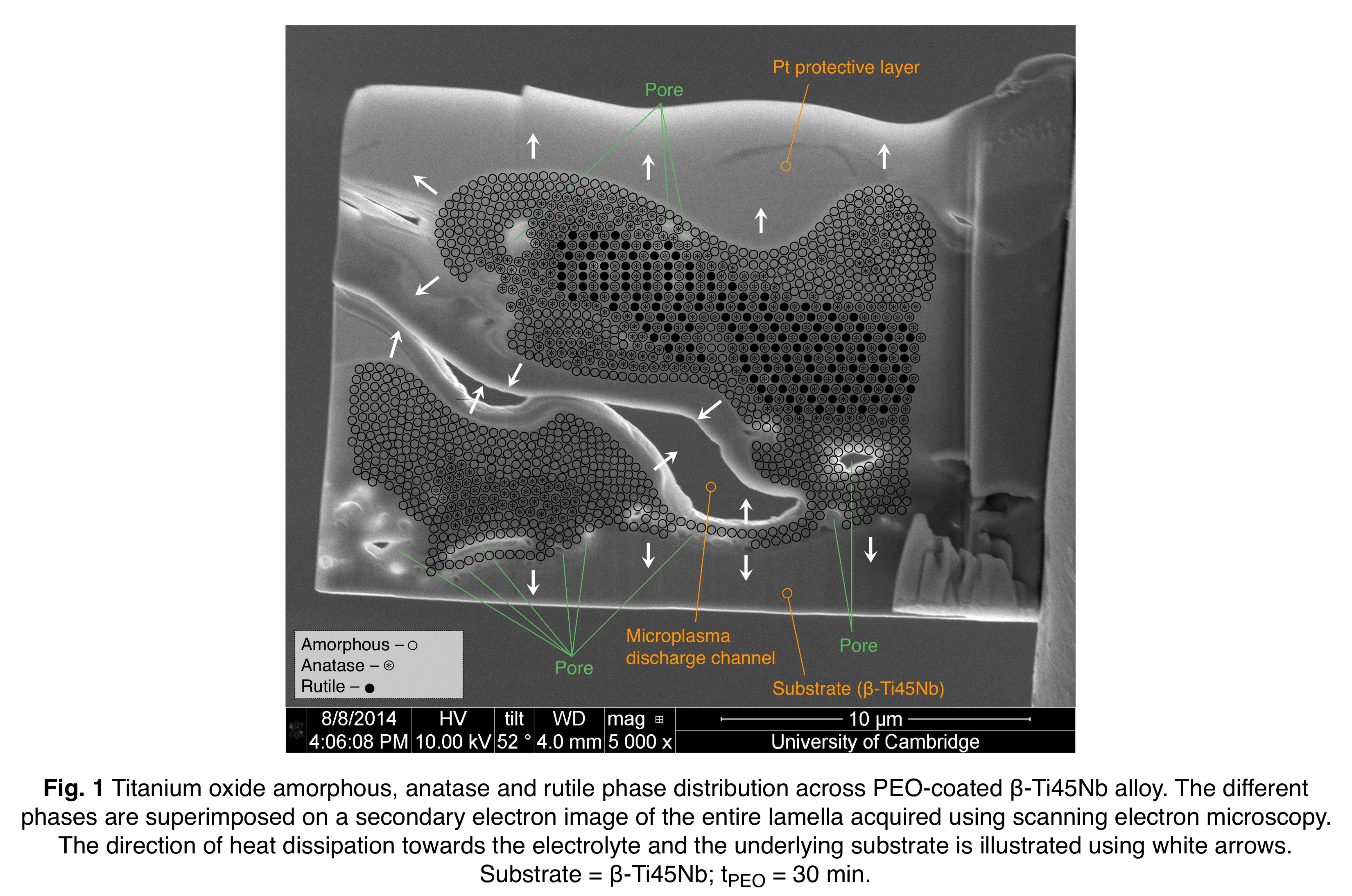
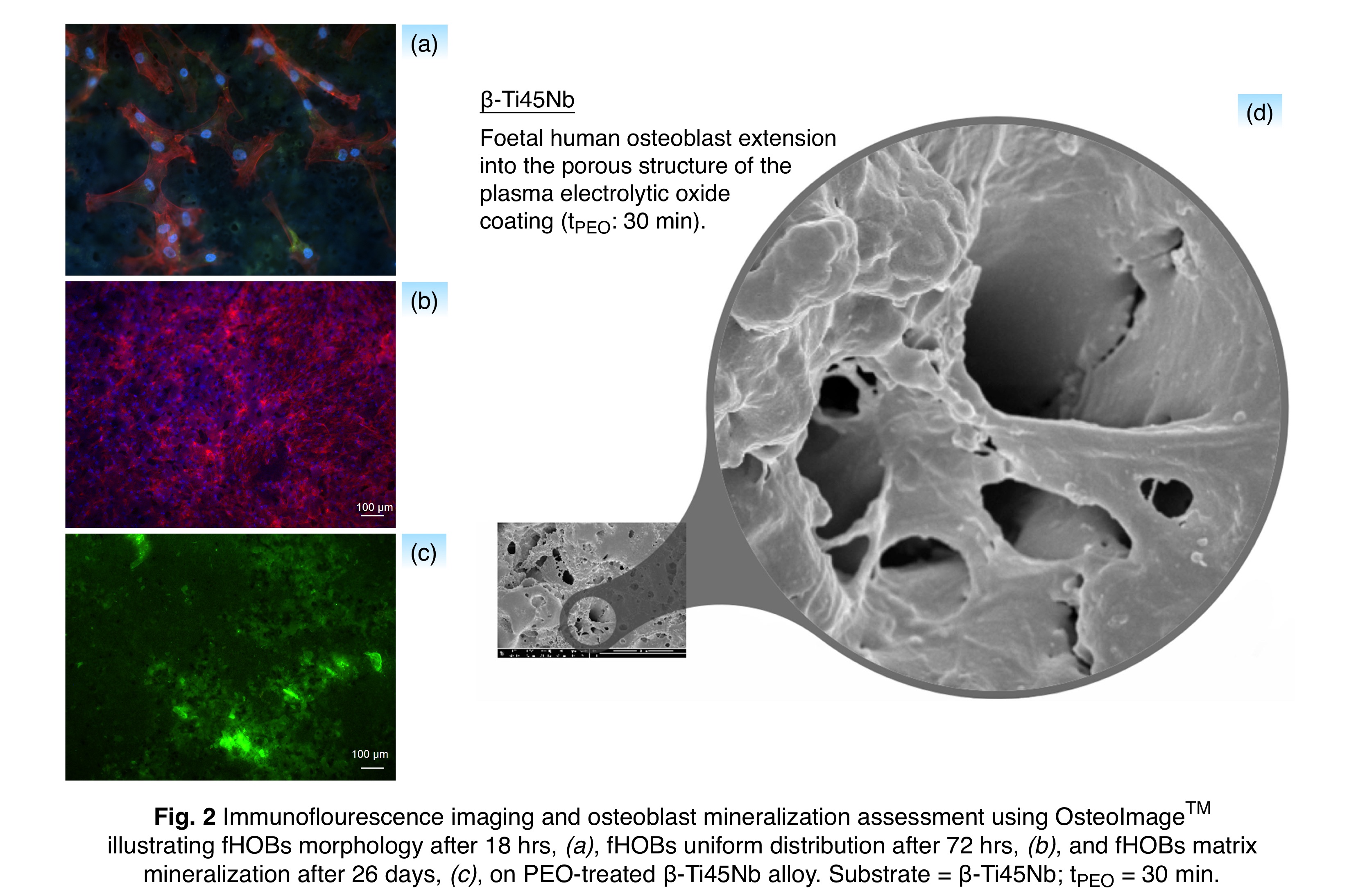
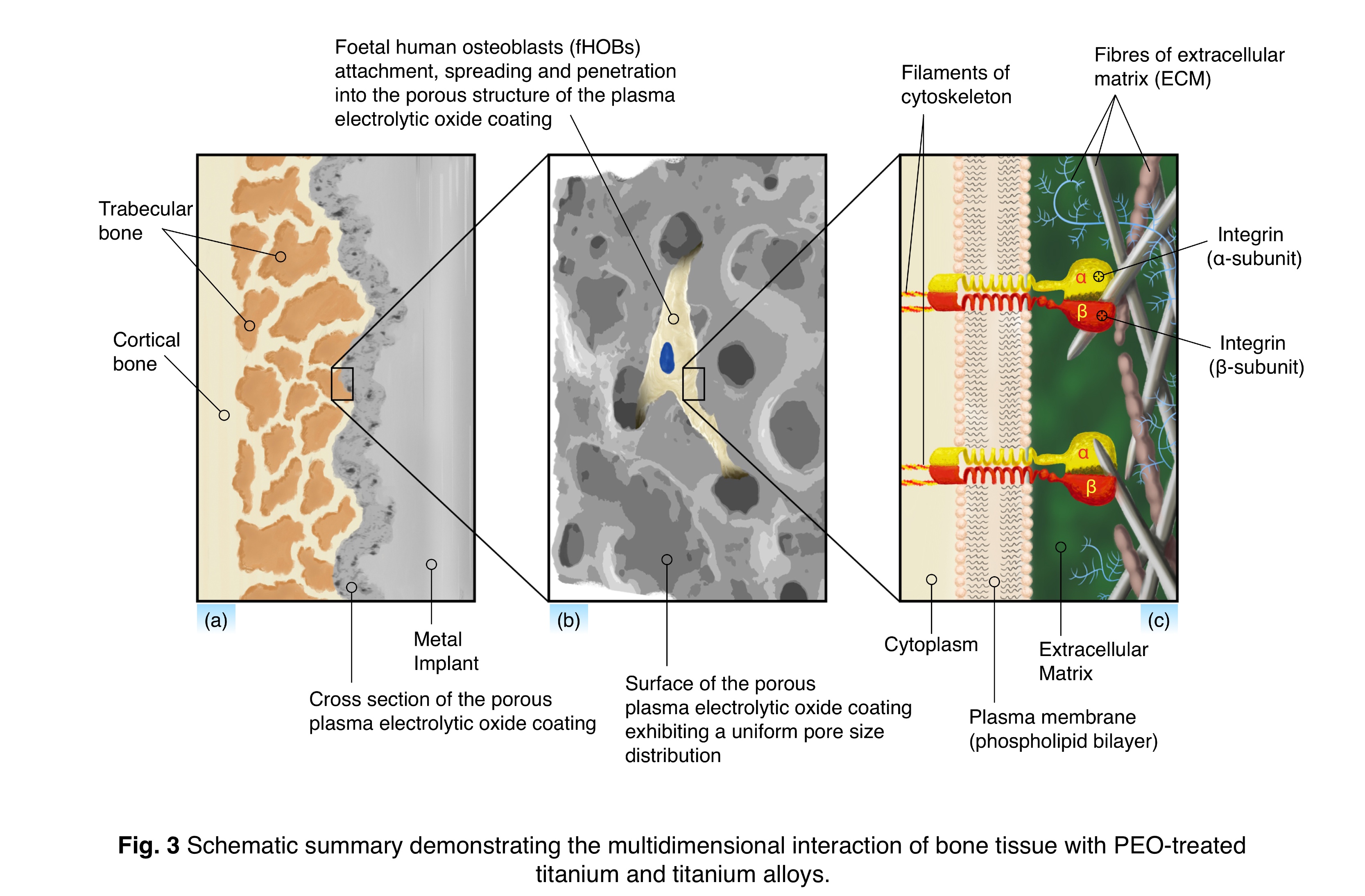
Conclusion: PEO was shown capable of producing anatase-rich, rough TiO2-based coatings with interconnected porosity on α-, (α+β)-, near β-, and β-type Ti-based alloys, Fig. 3. The surface physicochemical properties were found to depend on PEO processing time and substrate material. The study of cell attachment, metabolic activity, matrix formation and mineralization on all materials suggested that PEO application can be extended to modify the surface of low-modulus β-type Ti-based alloys offering a lower stress shielding effect, without impeding osteoblast response in vitro.
Authors would like to thank the Marie Curie Initial Training Network (Grant # 264635) for providing financial support.; A special thanks to Dr. J. Curran and Dr. C. Dunleavy for their assistance regarding PEO, to Mr. S. Collins, Mrs. F. Wisnivesky and Dr. G. Divitini for their expert guidance concerning TEM, and to Dr. V. Hoffmann and Dr. S. Oswald with regards to GD-OES and XPS analyses of the PEO coatings, respectively.
References:
[1] Geetha M. et al., 54:397-425, 2009
[2] De Jonge L.T. et al., 25:2357-2369, 2008
[3] Gittens R.A. et al., 32:3395-3403, 2011
[4] ZL Microdent, [Online] Available from: http://www.zl-microdent.de/?lang=en [Accessed: 7 April 2015]
[5] Nobel Biocare, [Online] Available from: https://www.nobelbiocare.com/uk/en/home.html [Accessed: 3 April 2015]
[6] Keystone Dental, [Online] Available from: http://www.keystonedental.com/ [Accessed: 7 April 2015]
[7] Hanada S. et al., 1284:239-247, 2005
[8] Uchida M. et al., 64:164-170, 2003