Statement of Purpose: Glaucoma is the second leading cause of blindness with more than 6 million blind eyes worldwide due to progression of the disease. InnFocus, Inc. (Miami, FL) has developed a Minimally Invasive Drainage Implant (“MIDI”) glaucoma device called the InnFocus MicroShunt® (Figure 1C) made from a highly biocompatible biomaterial called poly(styrene-block-isobutylene-block-styrene) or “SIBS” (Figure 2)[1]. SIBS has been used in human coronary arteries (Boston Scientific’s TAXUS® drug-eluting stent) for over 15 years[1]. Rabbit studies confirmed its superb biocompat-ibility in the eye[2]-[4]. This abstract summarizes the evolution of this device from clinical study outcomes.
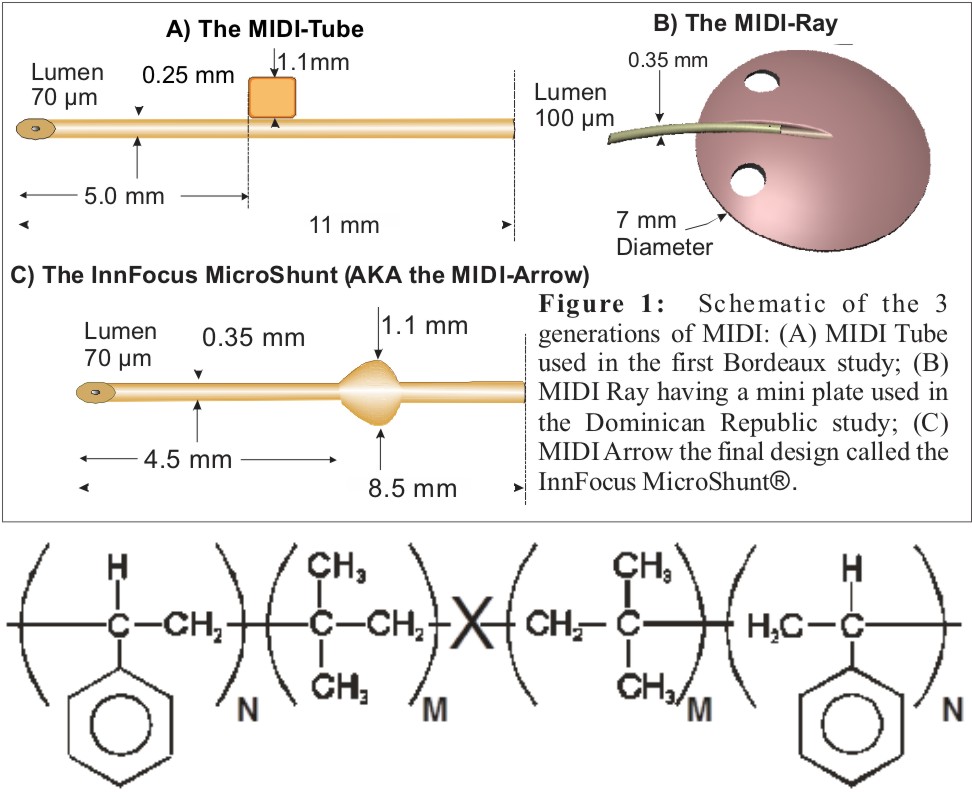
Figure 2: SIBS, where X is a hindered dicumylether and N/(N+M) = 0.3
Methods: SIBS (Shore 40A) was prepared according to the methods described by Kennedy.[4] The purified polymer was then extruded into tubes and compression molded. The lumen of the device serves as a flow restrictor to drop intraocular pressure (IOP). 3 Physical stability, hydrolysis, exhaustive extraction, leachable and biocompatibility testing were performed according to ISO and ANSI standards. The 3 iterations of glaucoma devices were implanted in patients at the Centro Laser, Santo Domingo, Dominican Republic and in Bordeaux, France and followed for two years. Patient selection was restricted to those with primary open angle glaucoma who had failed maximum tolerated glaucoma medication. All except MIDI Ray cases used the antiproliferative drug Mitomycin C (0.2-0.4 mg/mL applied for 3 min) intraoperatively as is used routinely in trabeculectomy.
Results: The device and material passed all pre-clinical tests. The table below summarizes the clinical data and surgical success rates of the various iterations of devices.
The InnFocus MicroShunt® was selected as the best iteration and the design was frozen. The one and two-year success rates, as measured by dropping IOP by ≥20% without surgical intervention, was 100% and 96%, the percent of patients totally off of glaucoma medication was 93% and 87%, the average IOP was 10.7 ± 2.8 mm Hg and 10.3 ± 2.0 mm Hg, which represents a 55% and 57% drop in IOP, respectively. There were no long-term sight-threatening adverse events.
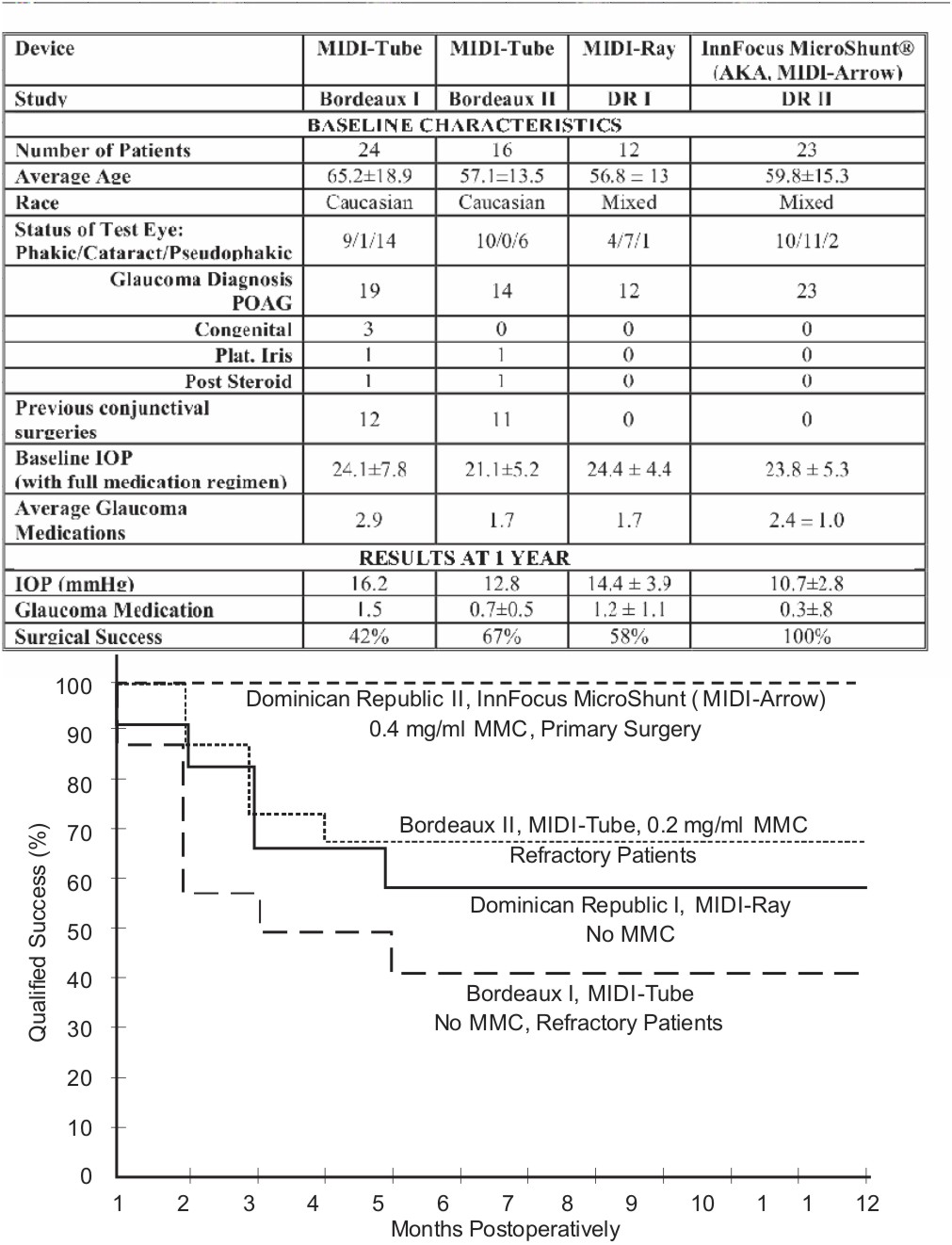
Figure 3: Kaplan Meier survival curves showing the qualified success rate of various iterations of the device.
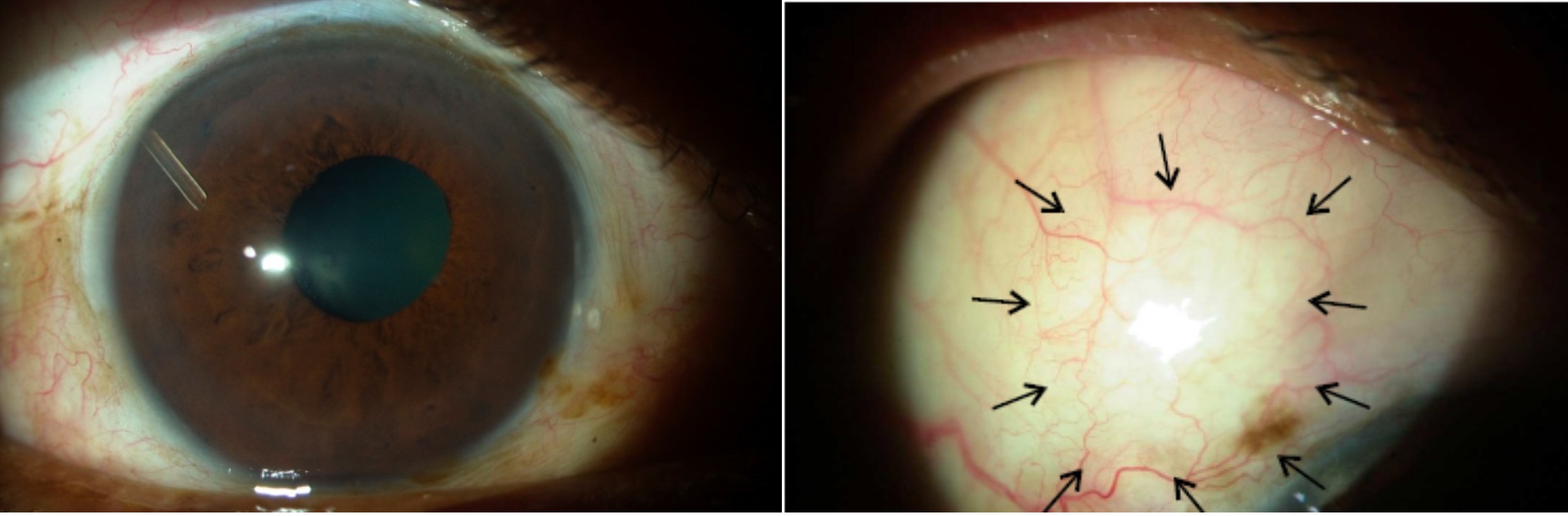
Figure 4: One year human implant of the InnFocus MicroShunt showing no encapsulation of the tube and a quiet diffuse bleb (arrows).
Conclusions: The InnFocus MicroShunt® made from SIBS is safe and effective.
FLEB, center grant NIH-P30EY014801, RPB, Lesieur Foundation (JMP), NIH-1R43EY018519-01A1, InnFocus, INC.; Maria Varela Consuelo, Sophie Abrespi and staff.
References:
[1] Pinchuk L, Biomaterials; 2008; 29(4); 448-460.
[2] Acosta AC, Arch Ophthalmol 2006, 124: 1742-1749.
[3] Arrieta -Quintero E, Ophthalmic Surg, Laser, Imaging 2011:42(4):338-45.
[4] Wang B, Polym Bull 1987;17:205–211.