Introduction: Tremendous strides have been achieved over the past several decades in the field of tissue engineering to construct implantable thin tissue constructs such as skin, cornea, and bladder[1]. However, obtaining functional, physiologically relevant tissues is still a major challenge due to the necessity of a vasculature system to supply nutrients and remove waste in thick constructs[2]. Additionally, tissue engineers have historically focused on fabricating tissue constructs containing a single vascular network. However, a key feature of complex biological systems, the presence of interpenetrating networks, such as the respiratory tree with its pervading vascular network, is central to mammal physiology yet has remained difficult to fabricate in vitro. To this end, we sought to develop a stereolithography (SLA)-based 3D printing system for fabrication of tissue constructs on the order of several centimeters containing complex interpenetrating networks.
Materials and Methods: Photorheological characterization of prepolymer formulations containing poly(ethylene glycol) diacrylate (PEGDA) and a photoinitiator lithium acylphosphinate (LAP) was performed to understand the gelation kinetics and modulus of a single layer of hydrogel. Various mathematical fractal-like, space-filling models, akin to physiologic vascular networks, were used to explore parametric designs with fine control of the geometry and architecture of multiple interpenetrating networks. Print fidelity was investigated by comparing designed models to volumetric renderings of micro-computed tomography (µCT) scans of printed gels. We added mixed populations of dsEGFP-expressing HEK293T, Luc2P-expressing HEK293T, and hMSCs into the prepolymer solution to encapsulate cells within the 3D hydrogel for assessing long-term survival in these complex engineered tissues.
Results and Discussion: The photorheological assay provided us with information on the exposure duration needed for a certain layer thickness based on parameters such as the photopolymer molecular weight, photopolymer concentration, initiator concentration, and light intensity for reproducible printing of multi-layered hydrogels with high z-resolution without delamination between layers (Figure 1A). µCT reconstructions of printed hydrogels demonstrated high printing fidelity with perfusable interpenetrating channels that are smooth and relatively round (Figure 1B). Bioluminescent and fluorescent images of printed hydrogels containing encapsulated cells demonstrated cell survival that was dependent on the printed network architecture.
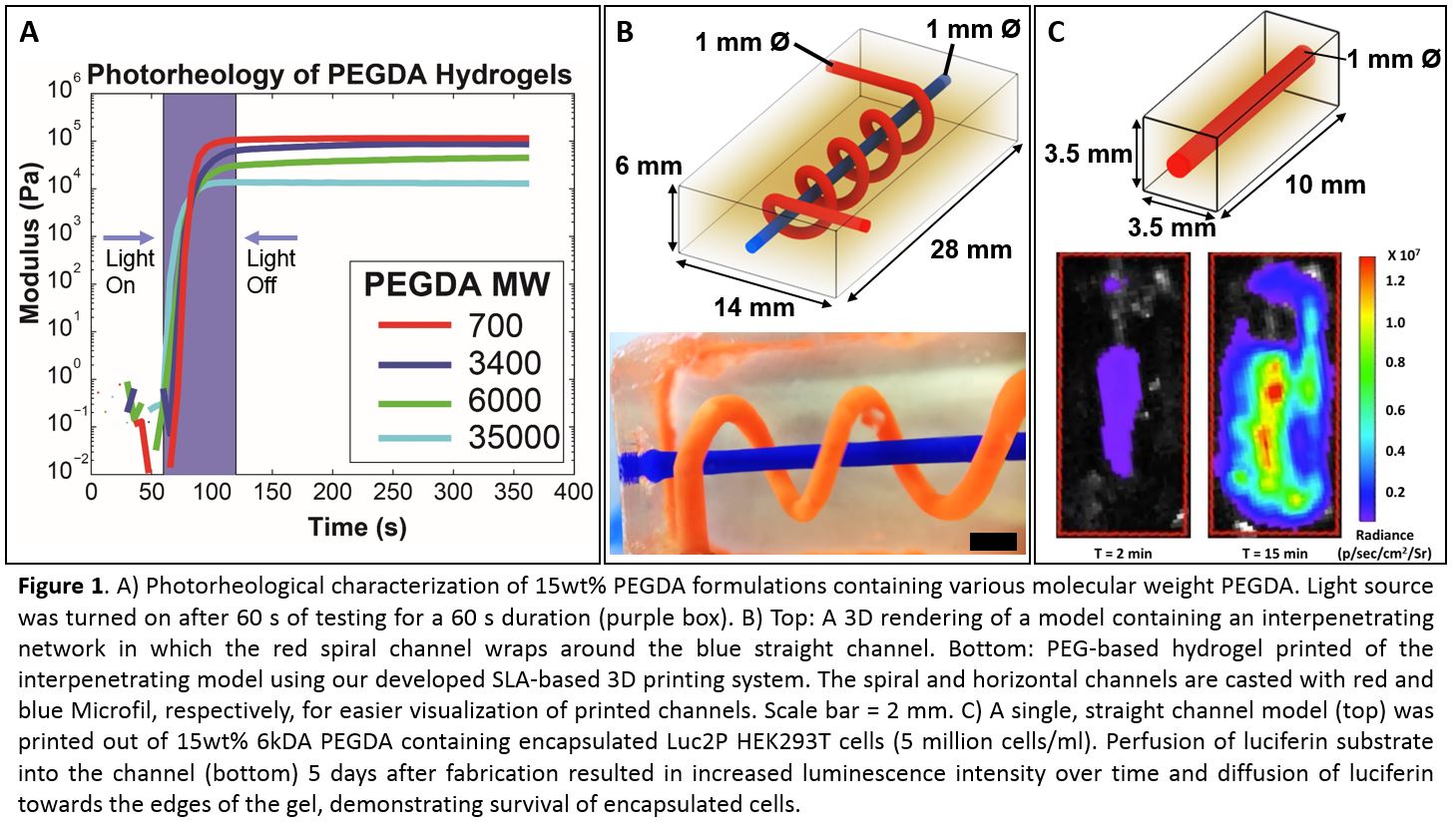
Conclusion: We have developed an open-source SLA-based 3D printer that allows for automatic fabrication of physiologically relevant sized hydrogels containing complex, perfusable interpenetrating micro-architectures in a solid gel. We expect the performed studies will provide insight to the field by demonstrating architectural features required to build living tissues the size of human organs.
Thanks to the Open Source Hardware Association, RepRap.org, and related projects and companies that support worldwide, open standardization of 3D printing. This work was supported in part by NSF Graduate Research Fellowship (BG).
References:
[1] Atala A, Kasper FK, Mikos AG. Engineering complex tissues. Sci Transl Med. 2012;4(160):160rv12. doi:10.1126/scitranslmed.3004890.
[2] Miller JS. The billion cell construct: will three-dimensional printing get us there? PLoS Biol. 2014;12(6):e1001882. doi:10.1371/journal.pbio.1001882.