Tracheal injury, stenosis, and malignancy demand surgical management or tracheal reconstruction [1],[2], however, the latter is an unmet clinical need as tracheal grafts and transplants fail due to the lack of a functioning epithelium and immune rejection. Tracheal immune rejection is largely associated with the epithelium, therefore we believe that the best method to optimize the use of donor tracheae is to decellularize only the epithelium and maintain the remaining tracheal structure, to replace it with a recipient-derived epithelium. We aim to develop a donor-recipient hybrid tracheal model by de-epithelializing the donor trachea and engrafting a pre-developed biomaterial-based recipient epithelial graft.
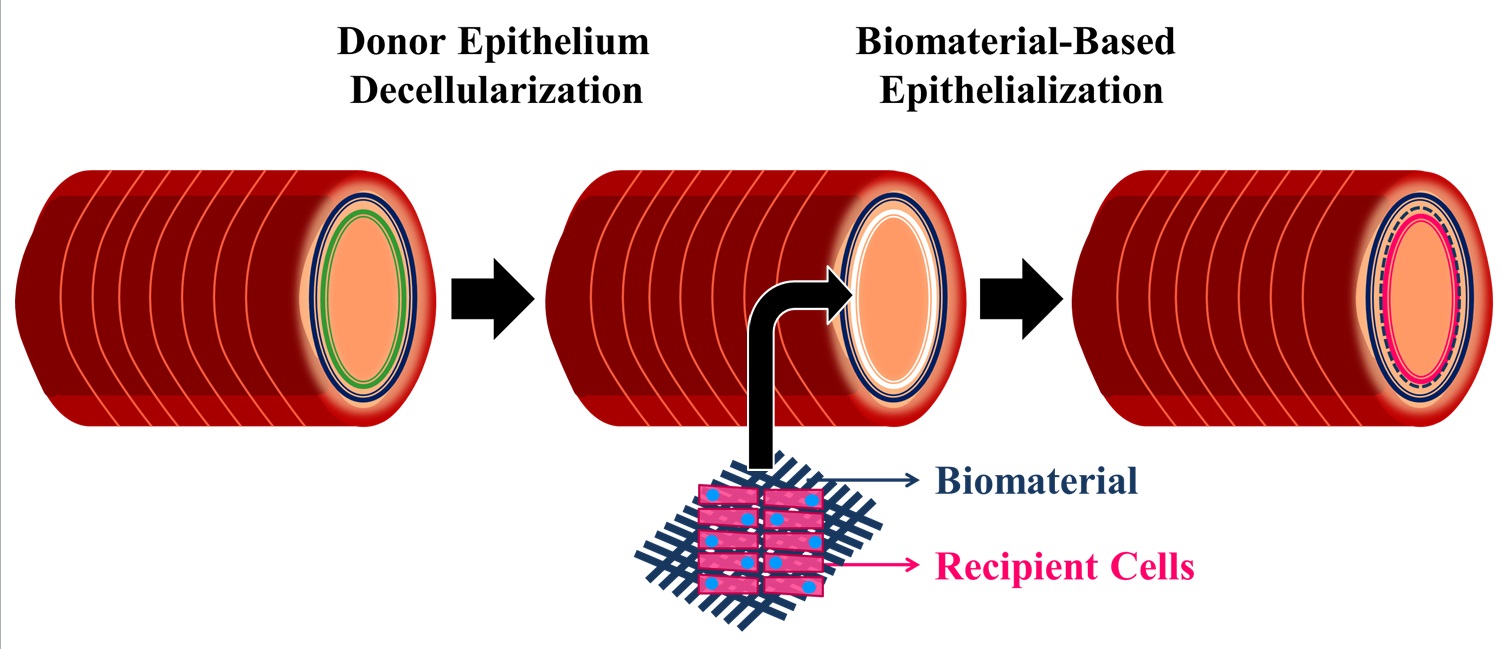
The absence of a functioning, ciliated, pseudostratified epithelium is a primary concern in tracheal regeneration. Clinically applied biomaterials demonstrate limited success in allowing proper airway epithelium growth and function [3]-[5]. Promising biomaterials have not been studied systematically. Therefore, we propose to perform the first comparative study to determine the optimal biomaterial for this context.
Hyaluronan-Poly-ethylene glycol (HA-PEG), Chitosan-Collagen, Collagen Vitrigel Membrane, Gelatin, Silk Fibroin, and Fibrin-PEG diacrylate, were screened to identify substrates that promote airway epithelium (cell lines: BEAS-2B, A549, CALU-3, and primary human tracheal epithelial cells: HTECs) attachment, viability and function while possessing mechanical strength. Cell viability and proliferation on the biomaterials are assessed through Live/Dead staining and Ki-67 immunocytochemistry, respectively, while cell attachment is assessed via focal adhesion kinase and vinculin immunocytochemistry. Tight junction (zonula occludins-1) formation and differentiation of HTECs into ciliated (acetylated α-tubulin) and secretory cells (mucin 5AC) in air-liquid-interface culture is examined via immunocytochemistry.
Preliminary results of phase contrast microscopy revealed no attachment of BEAS-2Bs and HTECs on HA-PEG hydrogels (HA-PEG, HA-PEG with functionalized IKVAV peptide, and HA-PEG with functionalized RGD peptide) by day 7. There was limited attachment of A549s and BEAS-2Bs and no attachment of CALU-3s on Chitosan-Collagen by day 14, and successful attachment with epithelial sheet formation with all cell types on Silk Fibroin by day 14 of culture. Irregular tight junction formation on HTECs on Silk Fibroin is detected as early as day 14 of air-liquid-interface culture and organizes by day 45.
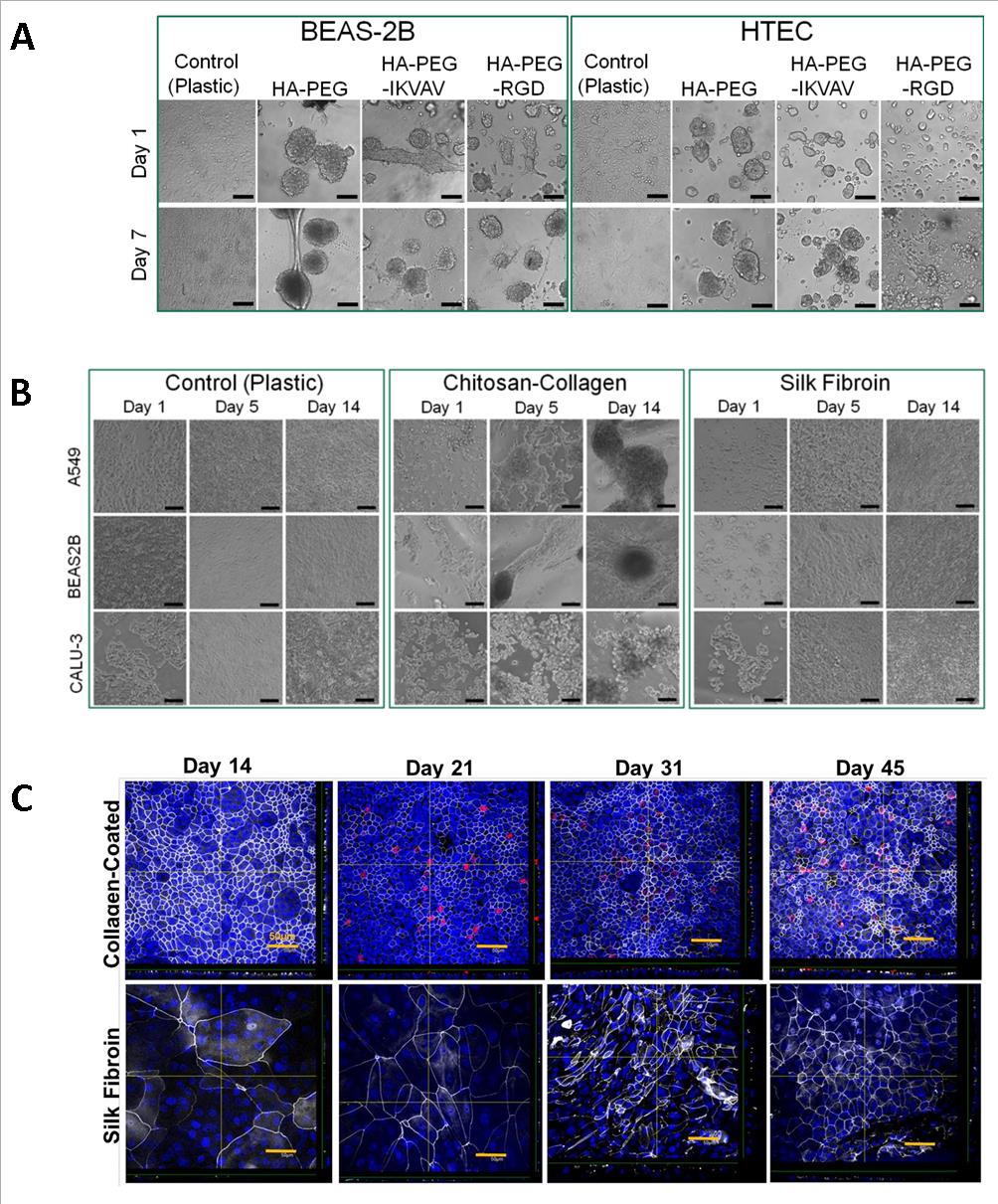
While Silk Fibroin is a promising biomaterial with exceptional mechanical strength [6], it demonstrates delayed maturation of HTECs and no ciliated cell differentiation by day 45 as opposed to day 21 on the Collagen I control. Silk Fibroin can be coated with other matrix proteins to promote enhanced epithelial cell attachment and differentiation. A 72-hour extracellular matrix screen of thirty individual or matrix protein combinations demonstrates BEAS-2B and HTEC attachment to Fibronectin (50 µg/ml) + Laminin (100 µg/ml) and RGD Peptide (50 µg/ml) + Laminin (50 µg/ml), and little attachment to Collagen I (200 µg/ml or 400 µg/ml) which is often used as a standard control.
With further investigation, an optimal biomaterial will be selected to develop an airway epithelium graft for human tracheal regeneration. This study will have significant clinical implications for viable tracheal transplants in future and can serve as a stepping stone for regenerating other organs comprising an epithelium.
Dr. Molly Shoichet; Alexander Baker
References:
[1] Grillo, H. C. (2002) Tracheal Replacement: A Critical Review. Ann Thorac Surg.73:1995-2004
[2] Fishman, J.M., Lowdell, M., and Birchall, M. A. (2014) Stem cell-based organ replacements – Airway and lung tissue engineering. Semin. Pediatr. Surg. 23:119-126
[3] Jungebluth P., Go T., Asnaghi A., Bellini S., Martorell J., Calore C., Urbani L., Ostertag H., Mantero S., Conconi M. T., and Macchiarini P. (2009) Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J. Thorac. Cardiovasc. Surg. 138:586-593
[4] Lin, C-H., Hsu, S-H., Su, J. M., and Chen, C-W. (2010) Surface modification of poly(ε-caprolactone) porous scaffolds using gelatin hydrogel as the tracheal replacement. J. Tissue Eng. Regen. Med. 5:156-162
[5] Paz, A. C., Soleas, J., Poon, J. C. H., Trieu, D., Waddell, T. K., and McGuigan, A. P. (2014) Challenges and opportunities for tissue-engineering polarized epithelium. Tissue Eng. Part B. 20:56-72
[6] Partlow B. P., Hanna C. W., Rnjak-Kovacina J., Moreau J. E., Applegate M. B., Burke K. A., Marelli B., Mitropoulos A. N., Omenetto F. G., and Kaplan D. L. (2014) Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 24:4615-4624