Introduction: Non-viral nanomaterials hold promise for use as siRNA delivery vectors for treating cardiac diseases. However, the delivery mechanisms of nanomaterials in heart have not been elucidated due to the difficulty to track and image nanoparticles in vivo. Here, we aim to investigate the efficiency, kinetics and distribution of siRNA delivered locally to zebrafish heart through nanomaterials. In particular, the cellular distribution of siRNA was studied via flk:GFP and cmcl2:GFP transgenic zebrafish models in which endothelial cells and cardiomyocytes express GFP, respectively.
Materials and Methods: A neutral polyethelenimine (PEI)-based material, PEI-HYD-RGD[1], and a dendrimer-based system, f-PAM4-PEG-R9, were synthesized through coupling reactions[2]. The materials were formulated with siRNA targeting Raldh2 (a retinoic acid-synthesizing enzyme) to form two types of nanoparticles, which were injected intrapleurally to zebrafish models with amputated hearts. The gene silencing effect was evaluated by qPCR. The uptake kinetics and the distribution of CY5-labled siRNA were analyzed via confocal microscopy of cryo-sectioned heart tissues.
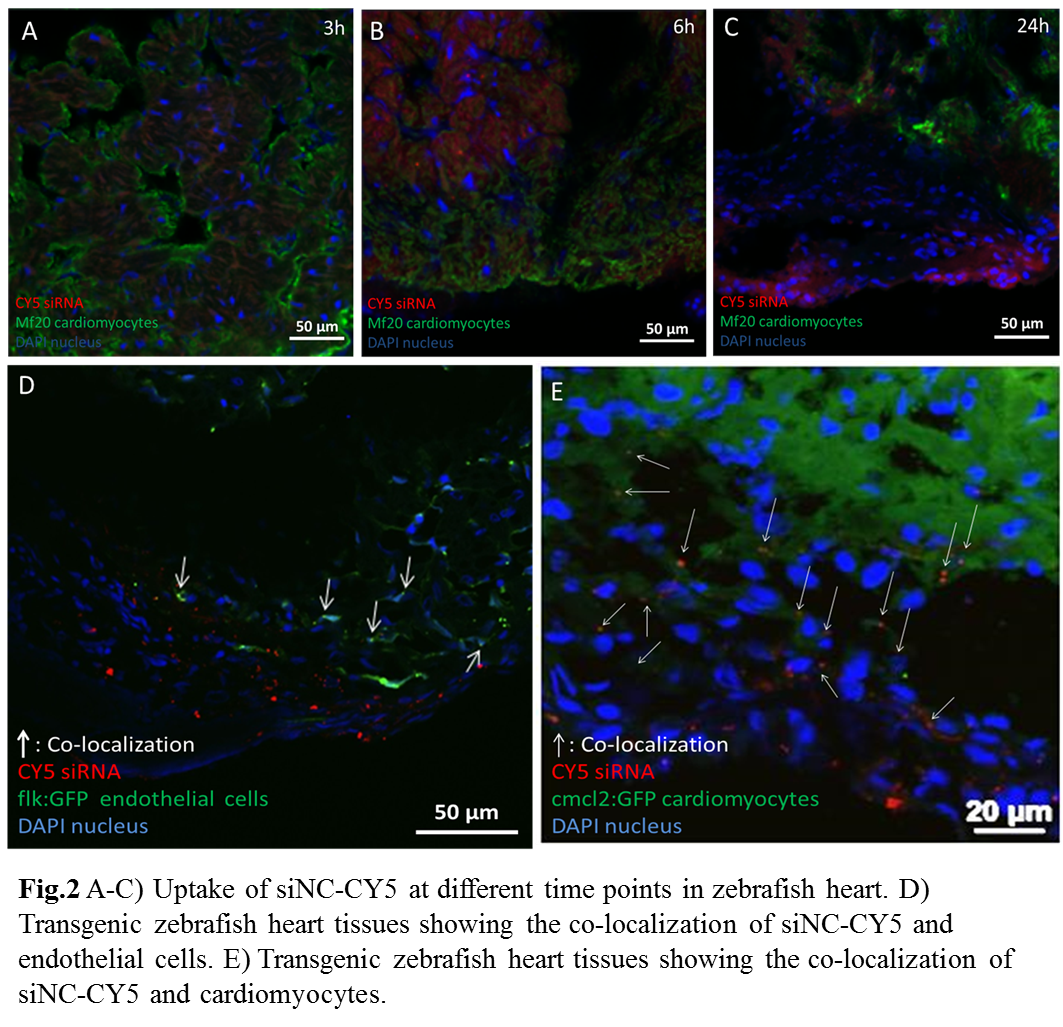
Results and Discussion: Both delivery systems showed gene silencing effects in zebrafish heart: The expression of Raldh2 was efficiently downregulated by about 50% and 80% via the PEI-HYD-RGD and f-PAM4-PEG-R9 vectors, respectively, compared to the models injected with DPBS only (Fig.1). In the f-PAM4-PEG-R9 system, the uptake of siNC-CY5 by the injured heart tissue began at 3 h after injection, reached the highest level in heart at 6 h and decreased at 24 h (Fig 2A-C). In the transgenic models, nanoparticle mainly located in the injured areas. In the PEI-HYD-RGD-medicated delivery, more nanoparticles were found associated with the cardiomyocytes (Fig 2E) than the endothelial cells (Fig 2D). The results indicate that nanoparticles may preferentially release therapeutic siRNA locally in injured heart tissues and induce differential distribution of siRNA in different cell types.

Conclusions:
Zebrafish provides a useful model to study the pharmacological mechanisms of siRNA in heart tissue. Nanoparticle-based system can effectively deliver siRNA in heart and may be further studied for optimal gene-silencing efficiency and siRNA distribution in vivo to develop new therapeutics for cardiac diseases.
References:
[1] Liu J, Zhou J & Luo Y, (2012). Bioconjug Chem, 23(2), 174-183.
[2] Liu J, Davis ME and Luo Y et al, (2013). Biomaterials, 34(14), 3729-3736.