Introduction: In the last years tissue engineering designed and developed several acellular substitutes based on tissue decellularization in the field of skin tissue regenerative medicine now widely provided for transplantation use[1]-[3]. Here we described the scientific design, realization and clinical use of human derived dermal matrix (HDM) in regenerative medicine
Experimental Methods: Procurement, decellularization and assessment of HDM properties: HDM was taken from the trunk area of multi-organ and/or multi-tissue donors following National Rules on harvesting, processing and distributing tissues for transplantation (CNT 19/06/2007) and was then decellularized at the Emilia Romagna Regional Skin Bank using a patented method (PTC/IB2008/002753). Microbiological and endotoxin analysis were performed to guarantee sterile conditions.
Histological analysis: Histological and ultrastructural analysis were performed on HDM as well as on skin biopsies of the patient. Samples were fixed with 10% formalin solution and paraffin embedded. After processing, histological sections (5-μm in thickness) were stained with hematoxilin and eosin (H&E). Alternatively, TEM analysis was performed on skin biopsies fixed in 2.5% glutaraldehyde buffered in phosphate 0.1M, post-fixed in osmium tetroxide 1% in cacodylate buffer, dehydrated and embedded in Araldite. Thin sections, stained with uranyl acetate and lead citrate were observed using a Philips 410 TEM.
Cell viability (MTT test): Using a 6-mm biopsy punch, six uniform samples were taken from HDM tissue. Two of them were soaked in liquid nitrogen for 10 minutes and used as negative controls. Tissue specimens were weighed, placed in a 12-well plate and incubated with 100 μl MTT solution for 3 h at 37°C in a 37°C incubator with 5% CO2. Each tissue punch was then placed in 1 ml dimethyl sulfoxide (DMSO) for 10 minutes. The solution was read on a spectrophotometer at 570 nm, and DMSO was assayed as the background. For each sample the viability rate was calculated as the ratio between the optical density (OD) at 570 nm and the weight in grams (gr). The viability index Iv(+) was defined as the mean of the viability rates of the four samples and was compared to the viability index Iv(-) of the two negative controls.
Clinical use: The HDM was applied on different clinical cases in which osteo-tendineous exposure as well as full-thickness skin wounds occurred.
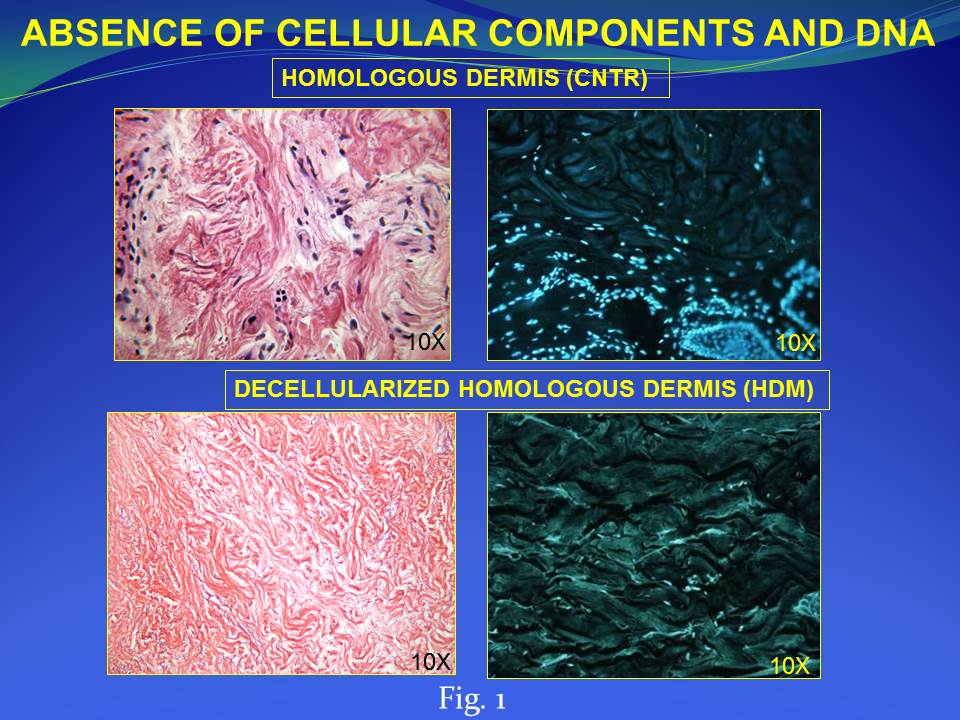
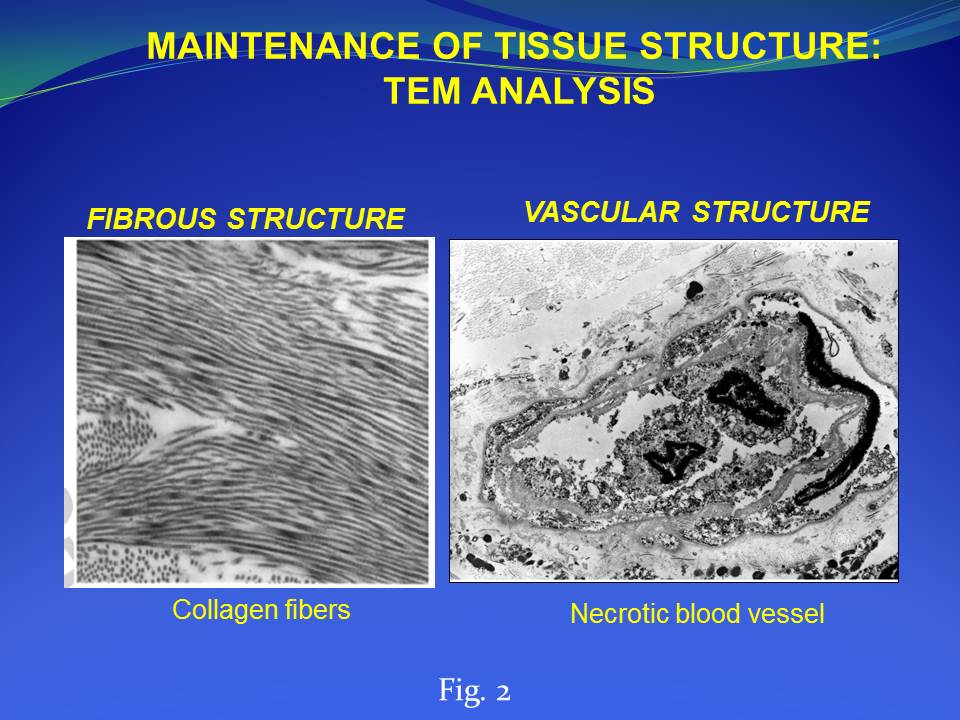
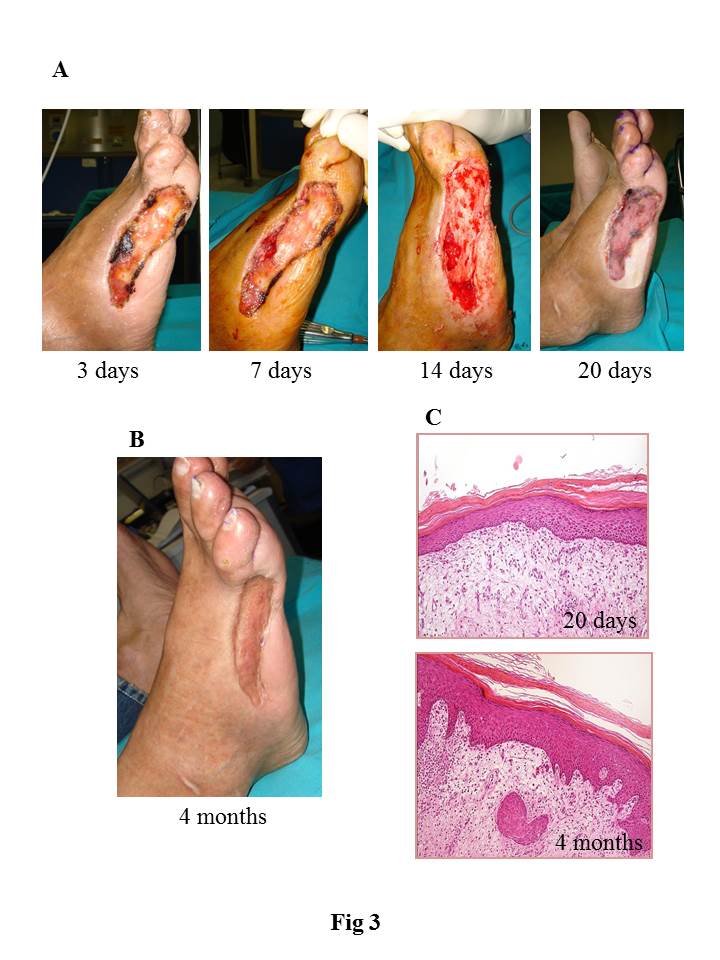
Results and Discussion: We developed and characterized a human derived dermal matrix. The use of HDM stimulates the healing process in the clinical case of full-thickness skin wound here described with a functional and aesthetic rescue of the damaged area observed during follow-up. An enrichment of skin-specific cell types after HDM treatment on the wound area was also identified by histological analysis.
Conclusions: Our results support the clinical use of HDM as a permanent dermal replacement for the treatment of full-thickness skin wounds for its ability to restore functional abilities and easthetic appearance through tissue regeneration of the damaged area rather than its replacement with scar tissue.
References:
[1] Fini M, Bondioli E, Castagna A, Torricelli P, Giavaresi G, Rotini R, Marinelli A, Guerra E, Orlandi C, Carboni A, Aiti A, Benedettini E, Giardino R, Melandri D."Decellularized human dermis to treat massive rotator cuff tears: in vitro evaluations" Connect. Tissue Res. 53:298 – 306, 2014
[2] Giavaresi G, Bondioli E, Melandri D, Giardino R, Tschon M, Torricelli P, Cenacchi G, Rotini R, Castagna A, Veronesi F, Pagani S, Fini M. "Response of human chondrocytes and mesenchymal stromal cells to a decellularized human dermis" BMC Musculoskelet Disord. 14:12, 2013
[3] Bondioli E, Fini M, Veronesi F, Giavaresi G, Tschon M, Cenacchi G, Cerasoli S, Giardino R, Melandri D. "Development and evaluation of a decellularized membrane from human dermis."J. Tissue Eng. Regen. Med. 8:325-336, 2014