Introduction: Type 1 diabetes mellitus is a chronic condition characterized by the autoimmune-mediated destruction of pancreatic β-cells[1]. Islets transplantation in the liver often results in limited islets survival presumably due to uneven islet distribution, abnormal blood flow dynamics and acute inflammatory response[2]. The objective of this study was to assess whether an antioxidant biodegradable citrate-based thermoresponsive hydrogel would support the viability and function of transplanted islets in a different location, specifically the epididymal fat pad.
Materials and Methods: Poly(polyethyleneglycol citrate-co-N-isopropylacrylamide) (PPCN) was synthesized from citric acid, poly(ethylene glycol), glycerol 1,3-diglycerolate diacrylate and poly-N-isopropylacrylamide[3]. The resulting polymer was functionalized with cell adhesion peptides or used to entrap stromal cell derived factor-1 (SDF-1). The angiogenic capacity of SDF-1-releasing PPCN was evaluated in a diabetic mouse wound healing model. Islet viability was evaluated in vitro using a Live/Dead Assay and the islets function was assessed using the streptozotocin-induced diabetic mice model. Briefly, 200 mouse islets were isolated from a syngeneic donor and either: a) suspended in the PPCN solution and applied onto the epididymal fat pad or b) directly transplanted into the kidney capsule (control). Non-fasting glucose levels were monitored over time to assess the performance of the islets post-transplantation.
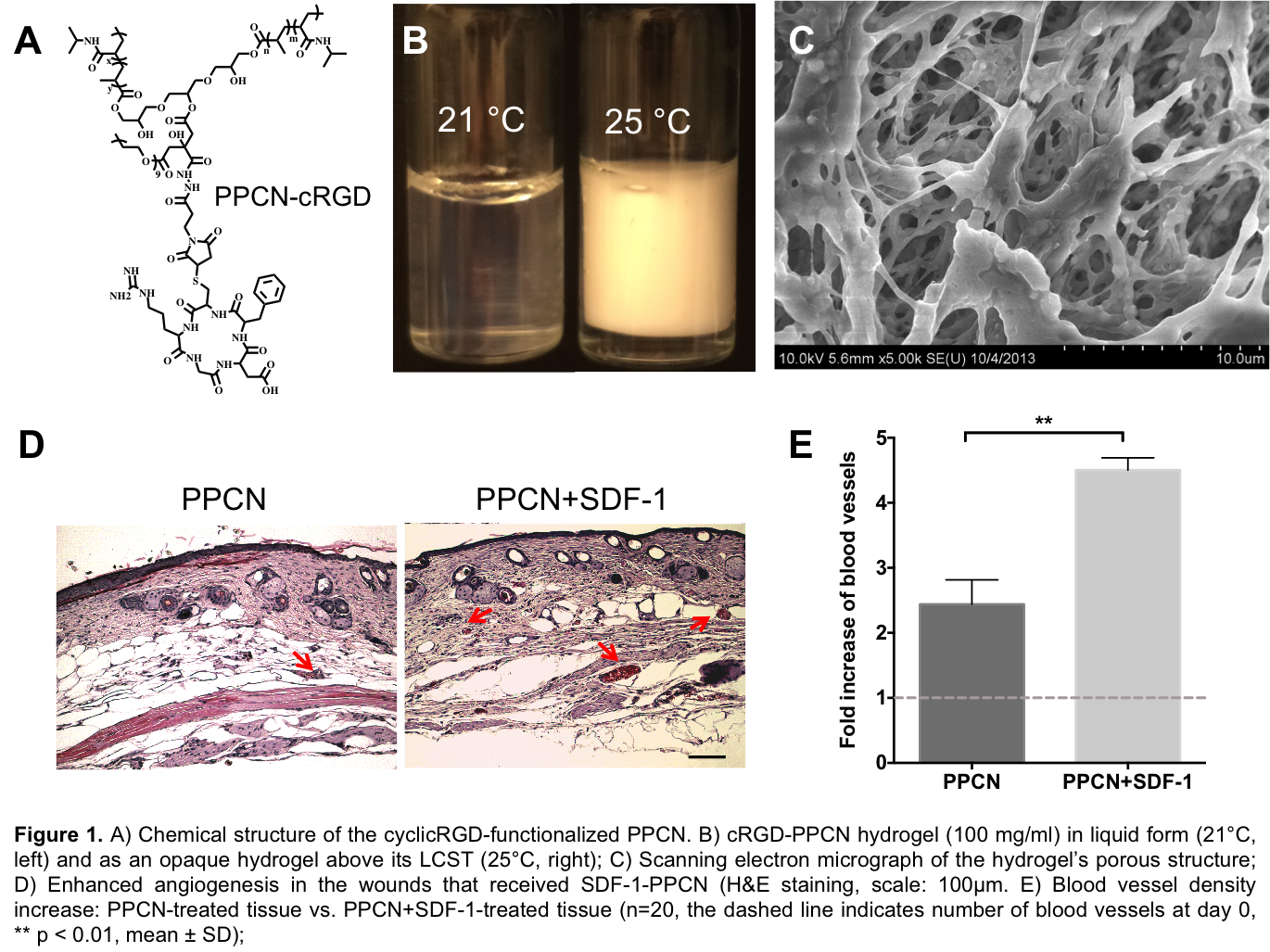
Results: Cyclic-RGD (cRGD) was covalently linked to PPCN and the cRGD-PPCN retained its ability to reversibly change between liquid and gel phases at a lower critical solution temperature (LCST) of 25°C and maintain micro- and macroporosity within the gel phase. SDF-1 was entrapped and slowly released from PPCN. Wounds treated with SDF-1-PPCN demonstrated enhanced vascularization.
Ten days after isolation, Islets entrapped within PPCN maintained their original morphology and viability in vitro. Islets entrapped in cRGD-PPCN restored euglycemia (glucose<200 mg/dl) within an average of 1.8 (±0.8) days following transplantation. Euglycemia was maintained in the cRGD-PPCN and control group for the duration of the experiment (37 days).
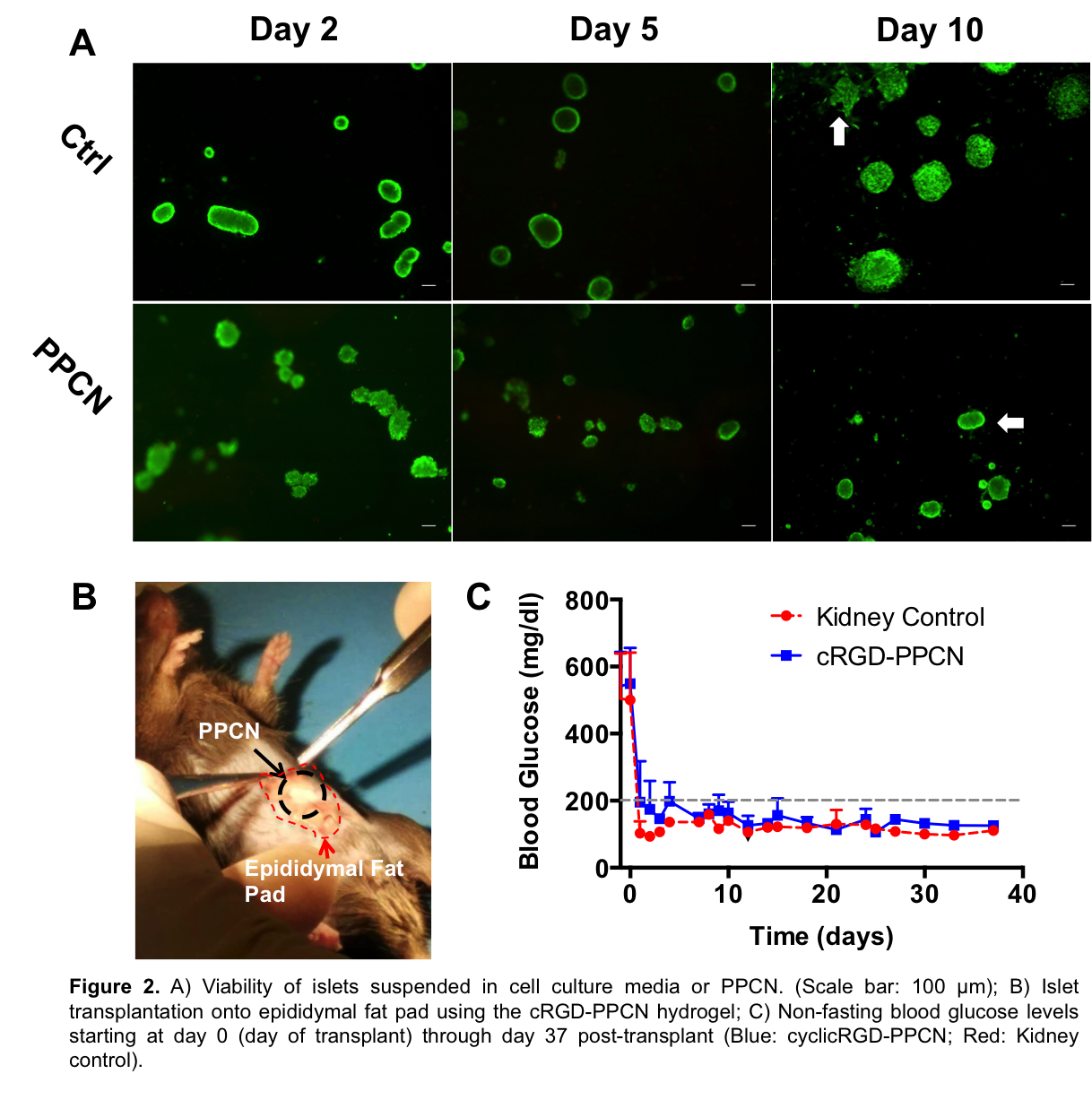
Discussion: In this study, we demonstrated the ability for SDF-1-PPCN to induce angiogenesis in diabetic mice, setting the foundation for using this system to maintain islet function in large animal models. In vitro, PPCN preserved the normal islet morphology while islets cultured in cell culture media began to loose this morphology due to cell spreading. The negative charge and hydrophilicity distribution within PPCN as well as its biocompatibility (low to no inflammation) likely created an environment that supports the viability and function of the islets, allowing successful extrahepatic islet transplantation in this mouse model. The observed restoration of euglycemia is superior to what has been reported for other hydrogels when taking into account the number of islets required to achieve euglycemia and the time it took to reach the euglycemic state[4]-[7].
Conclusion: These findings confirm that a novel citrate-based thermoresponsive hydrogel can serve as a platform vehicle for extrahepatic islet transplantation. PPCN allows the modification of the islet microenvironment to achieve functional vascularization and display peptides such as cRGD if needed, thus enhancing long-term islet survival and function.
References:
[1] Alberti, K. & Zimmet, P. Definition, Diagnosis and Classification of Diabetes Mellitus and its ComplicationsPart 1: Diagnosis and Classification of Diabetes MellitusProvisional Report of a WHO Consultation. Diabetic Medicine 1–15 (1998).
[2] Ryan, E. A. et al. Five-Year Follow-Up After Clinical Islet Transplantation. Diabetes 54, 2060–2069 (2005).
[3] Yang, J., van Lith, R., Baler, K., Hoshi, R. A. & Ameer, G. A. A thermo-responsive biodegradable polymer with intrinsic antioxidant properties. Biomacromolecules 15, 141008122143009 (2014).
[4] Stendahl, J. C., Wang, L.-J., Chow, L. W., Kaufman, D. B. & Stupp, S. I. Growth Factor Delivery From Self-Assembling Nanofibers to Facilitate Islet Transplantation. Transplantation 86, 478–481 (2008).
[5] Liao, S. W. et al. Maintaining functional islets through encapsulation in an injectable saccharide–peptide hydrogel. Biomaterials 34, 3984–3991 (2013).
[6] Phelps, E. A., Headen, D. M., Taylor, W. R., Thulé, P. M. & García, A. J. Vasculogenic bio-synthetic hydrogel for enhancement of pancreatic islet engraftment and function in type 1 diabetes. Biomaterials 34, 4602–4611 (2013).
[7] Blomeier, H. et al. Polymer Scaffolds as Synthetic Microenvironments for Extrahepatic Islet Transplantation. Transplantation 82, 452–459 (2006).