Introduction: Guided Bone Regeneration (GBR) has reached the state of standard care in Dental Implantology. In this procedure, a bone conductive matrix is placed in the defect site and a cell barrier membrane is then placed over the bone conductive matrix to prevent the infiltration of epithelial and connective tissue cells. Clinical outcomes from this procedure have been reproducible and bone regeneration is generally satisfactory for implant placement in 4 to 6 months. It is desirable that the bone conductive matrix be resorbable, porous, having a structure similar to the native bone mineral to guide and support bone growth and facilitate in vivo remodeling. In part I of this series we present the development and in vitro characterization of a newly developed Porcine Anorganic Bone Mineral (PABM) as a bone conductive matrix to guide bone regeneration in GBR procedures. Results of in vivo study in a canine intrabony defect model as well as human case studies are presented in Part II.
Materials: PABM was isolated from epiphysis of the porcine femoral head. The adhering tissues were removed and the bone was ground into particles. After thorough rinsing with hot water (90-100°C) to remove blood and water soluble materials, the particles were subjected to a combination of chemical and heat treatment to remove organic moieties without significantly change the native mineral structure. The PABM thusly prepared was ground, sieved, packaged and sterilized.
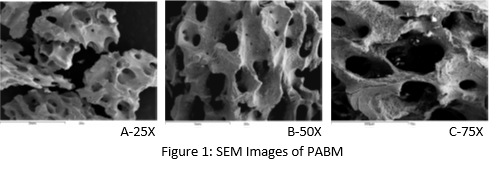
Methods: SEM: Micrographs were recorded using a scanning electron microscope (JEOL Ltd. JSM 6100). Pore size was measured from SEM images.
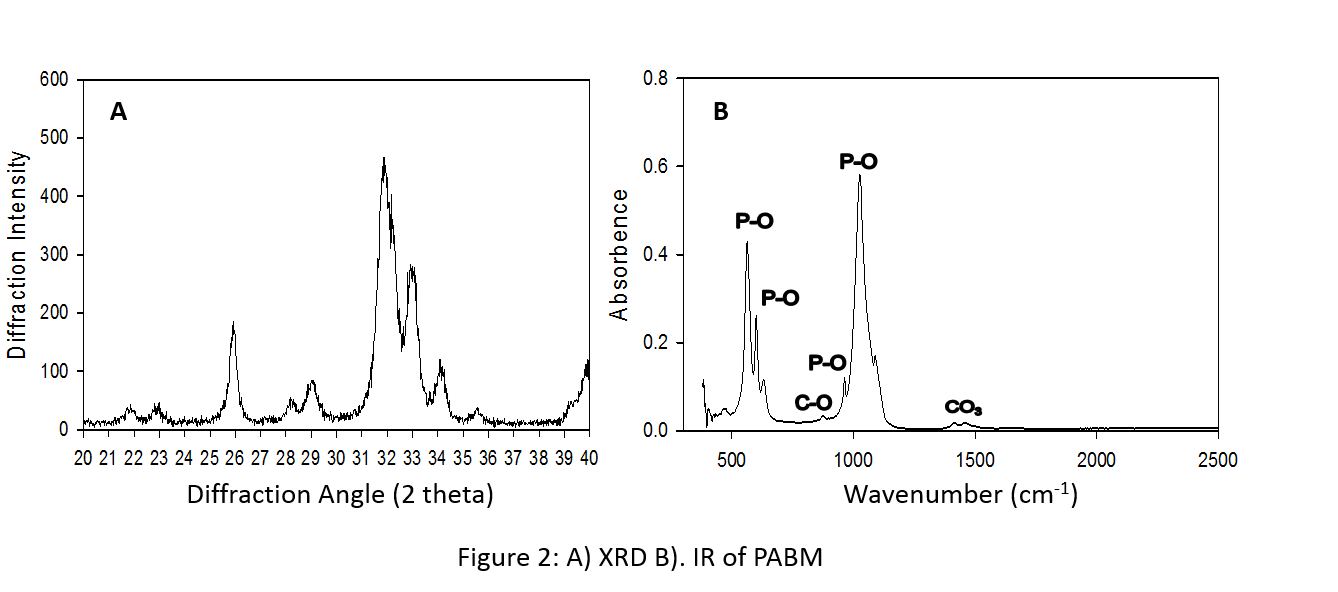
X-Ray Diffraction (XRD): Analysis was conducted using PHILIPS PW 1710 X-ray diffractometer to obtain key reflections for the identification of mineral structure.
FTIR Spectroscopy: Samples were analyzed using Perkin Elmer 983G FTIR spectrophotometer to determine the functional groups.
Volume Fill Measurement: A unit weight of mineral material was poured into a small volumetric cylinder and the volume occupied by the mineral was recorded and expressed as cm3/g.
Results and Discussion: Fig. 1 revealed the porous structure and rough surface appearance of PABM. The majority of macropores are in the range of 0.1mm to 1.0mm. These properties are favorable for cell adhesion and bone conduction. Fig. 2 showed the results of XRD and FTIR spectroscopy performed on PABM. The XRD diffraction pattern is close to the mature native bone diffraction pattern of apatite nature with low degree of crystallinity [1]. The major IR absorption peaks for PABM is consistent with bone mineral structure containing carbonate, including the phosphate ion bands, carbonate ion (CO3) band, and the carbonyl (CO) band. Results from volume fill measurement (Table 1) indicates that PABM provides ample intra- and inter-particle space for osteoconduction and new bone deposition. The percent volume fill decreases with decreasing particle size as some of the pores have lost from the change in particle sizes.

Conclusion: The PABM retains the natural mineral structure. It can provide a high degree of osteo-conductivity and yet minimizes the amount of implant material left behind post bone regeneration. These properties are desirable for GBR procedures.
References:
[1] Tadic D. and Epple M., Biomaterials. 25(6): 987-994, 2004