In recent years, magnesium and its alloys have received much attention as a new biomaterial in orthopedic applications due to their biodegradability, biocompatibility, and their mechanical properties that are similar to natural bone. The most common problem associated with magnesium as a biomaterial is low corrosion resistance in physiological solutions. This decreases the mechanical integrity of the implants in the early stages of healing and has a negative impact on the overall biocompatibility. Coatings can be used to control the degradation rate and provide optimum biocompatibility of these implant materials. Mesoporous silica materials have been shown to have good bioactivity and the ability to stimulate osteoblast proliferation and differentiation at implant surfaces. Furthermore, they have been shown to be non-toxic and non-inflammatory to mammalian tissues.
Materials and Methods: The surfaces of Mg AZ31 alloys were prepared by polishing the samples to one micron surface finish and then cleaning them by sonication in acetone for 20min. alkaline aging was used to promote the formation of hydroxyl grope on the surface which is important for the covalent bond formation between the metal and the coating. Two types of coating were used in this research: silane coating as a protective film and mesoporous silica coating. The silane coating solution was prepared by using methanol as a solvent, deionized water, ammonia, and TEOS in varying v/v ratio and then hydrolysis for 24h and they were deposited for varying of time. The mesoporous silica coating solution was synthesized by using TEOS as a silica precursor and cationic surfactant C12CAT (alkyltrimethylammonium chlorides), deionized water, methanol, and ammonia. The molar ratio of TEOS: C12CAT: deionized water, methanol, and ammonia was 1:0.4:774:1501:72. The solution was hydrolysis for 1h before coated the samples by spin Coating. The samples were dried and curing for 1h after each coated step. To remove the surfactant, the coated samples were calcined at 350 °C for 3h. These mesoporous silica films will be further modified through deposition of calcium phosphate to produce self-healing. Attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and atomic absorption spectrometry (AFM) were used to characterize the surface of coated sample before and after calcination.
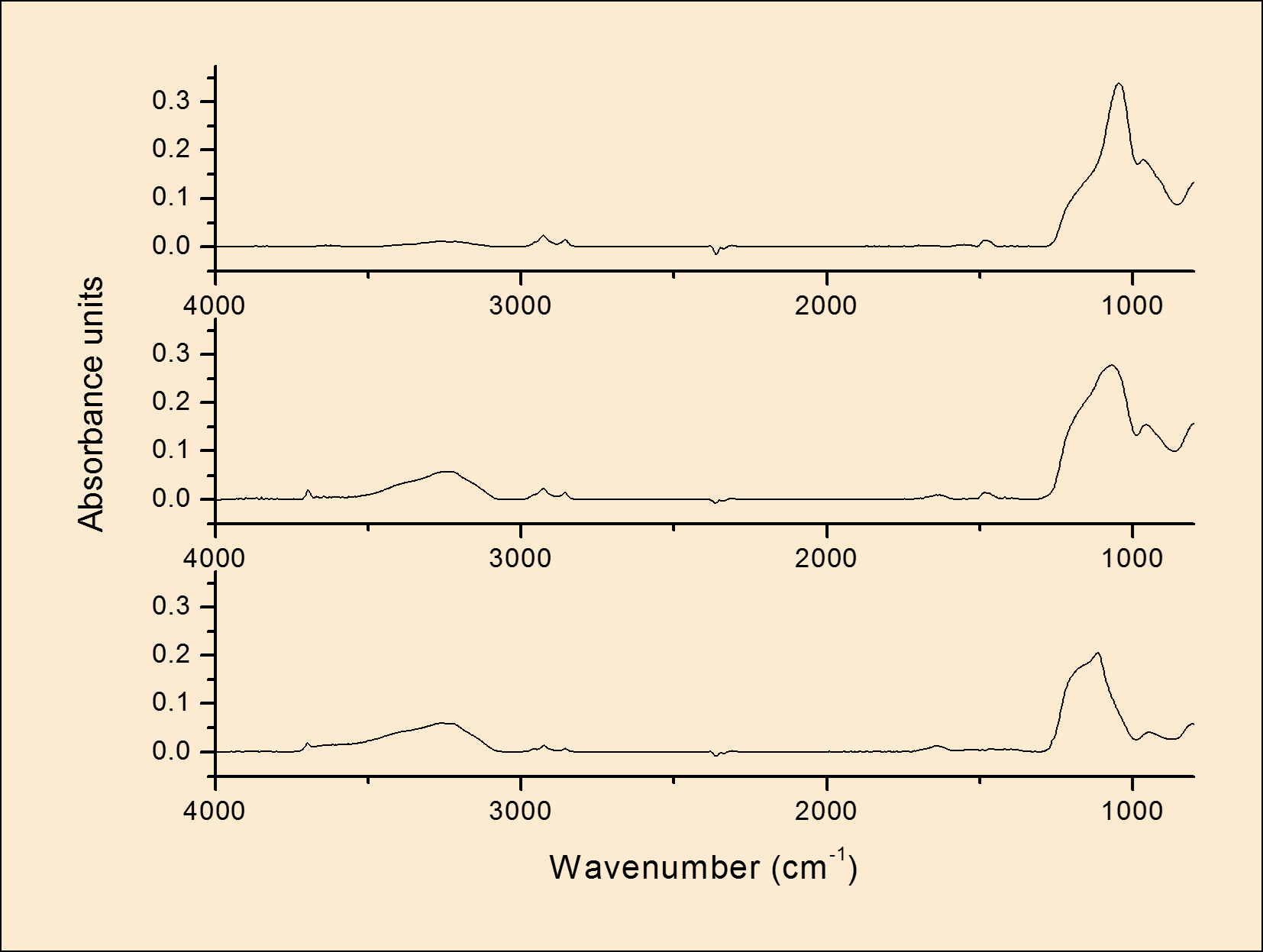
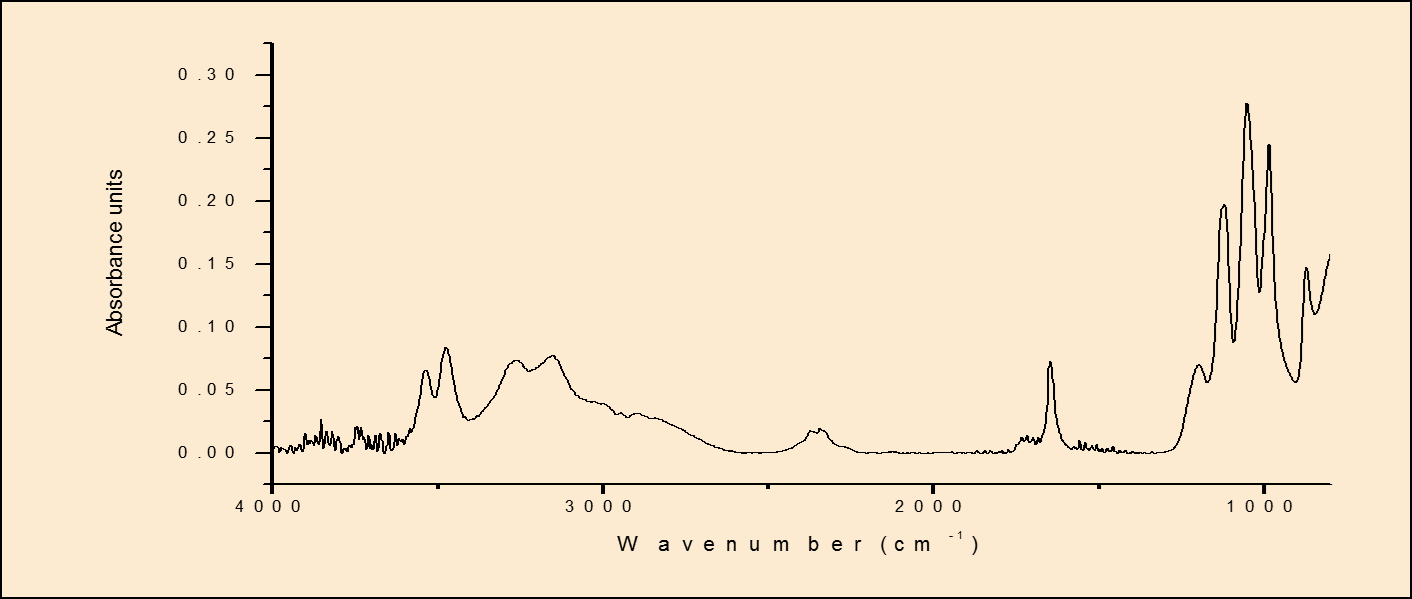
Disscution: The initial results show that with the right conditions, it is possible to deposit mesoporous silica particles on Mg alloys. However, corrosion due to the presence of surfactant in the coating bath was observed. This problem was addressed by the introduction of a silica-based pre-layer to protect the surface during mesoporous silica film formation. The influence of coating bath concentration and deposition time on the pre-layer thickness and uniformity were investigated. From (Fig. 1.) it is clear that deposition time does not have significant effect on TEOS film. However, the thickness of the TEOS film increases with increase the TEOS concentration. So, the best conditions to deposit TEOS layers were 20 min as the optimum deposition time and the 3.2% as the optimum concentration. IR Spectra for as deposited mesoporous silica at various deposition times (the results are not shown) indicated that short deposition time was the best for the coating so the 20 min was chosen as the best deposition time for the mesoporos silica film. The thickness of the film was increased with increase the number of layers as shown in (Fig.2.). Moreover, the AFM images (the results are not shown) indicated the presence of the spherical particles on the film and increase the density of the film with increase the number of layers. Therefore, to get the mesoporous silica film, the surfactant was removed completely after calcination. (Fig.3.) Calcium phosphate (CaHPO4.2H2O) is successfully deposited on the mesoporous silica film on Mg alloys.

Laurentian University; Ministry of Education in Saudi Arabia
References:
[1] Leali, P. T., and Merolli, A. "Fundamentals of Biomaterials." Biomaterials in Hand Surgery. Milan: Springer (2009): 1-11.
[2] Shaw, B.A. Corrosion resistance of magnesium alloys: Handbook. ASM International 13A (2003): 692-696.
[3] Boissière, C., Larbot, A., and Prouzet, E. "Synthesis of mesoporous MSU-X materials using inexpensive silica sources." Chemistry of materials 12.7 (2000): 1937-1940