Introduction: Osteoarthritis (OA) is a growing issue worldwide, yet many of the clinical treatments for OA are only palliative[1]. Since osteochondral (OC) defect repair remains a significant problem in orthopedic surgery, researchers are currently working to develop matrix systems for OC repair and regeneration[2]. While some success was reached using single and bi-phasic designs, overtime, the need for more tailored systems became apparent[3]-[5]. Our group has introduced a graded structure with bone and cartilage supporting layers arranged in reverse gradients in order to achieve an integrated OC matrix system. We hypothesize that by carefully tailoring each biomaterial phase we can not only support regeneration of the bone phase, but also provide the cues necessary to promote regeneration of the cartilage phase. Here, we aim to design and investigate the hydrogel phase with varying mechanical strength to support cartilage regeneration, and explore the gel’s integration into the polymeric phase creating a new generation scaffold system for OC defect repair and regeneration.
Materials and Methods: Using the Hystem kit (ESI BIO), a hyaluaronan gel (HA) was mixed with a polyethylene glycol (PEG) cross-linker in ratios of 1:1 – 1:7 (PEG:HA), and characterized for storage/loss modulus using a rheometer. After a range of gels were selected based on their mechanical profiles (1:2, 1:4, and 1:7), 400-500k mesenchymal stem cells were seeded in the gels and cultured for 21 days in chondrogenic media. After 21 days the GAG content of the samples were determined through alcian blue staining as well as the DMMB assay. Additionally, immunofluorescence (IF) staining was done to observe collagen II, aggrecan, and Sox9. Finally, using thermal sintering and porogen leaching, poly(85 lactide-co-15 glyclolide) (PLGA) microspheres were fabricated into a gradient scaffold which was infiltrated with the selected gel prior to μCT analysis[6].
Results and Discussion: Based on the rheological data it was determined that modification of the PEG:HA ratio can vary the overall modulus of the gels (~10Pa -45Pa). After 21 days of culture the results show that with an increase in modulus there is also an increase in GAG expression and presence of key proteins such as collagen II and aggrecan (Figure 1).
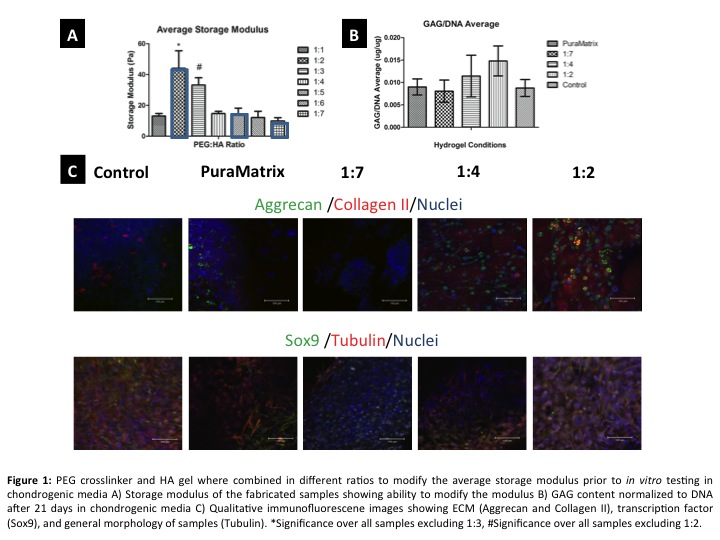
The time sensitive crosslinking of the HA gel allows it to infiltrate the available pore space of the gradient matrix prior to full gelation, acting as a chondro-inductive secondary phase/cell delivery vehicle. This said, as supported by the μCT data, when the designed gel is used to infiltrate the pore space of the matrix we find that there is in fact an inverse gradient of hydrogel to polymer (Figure 2).
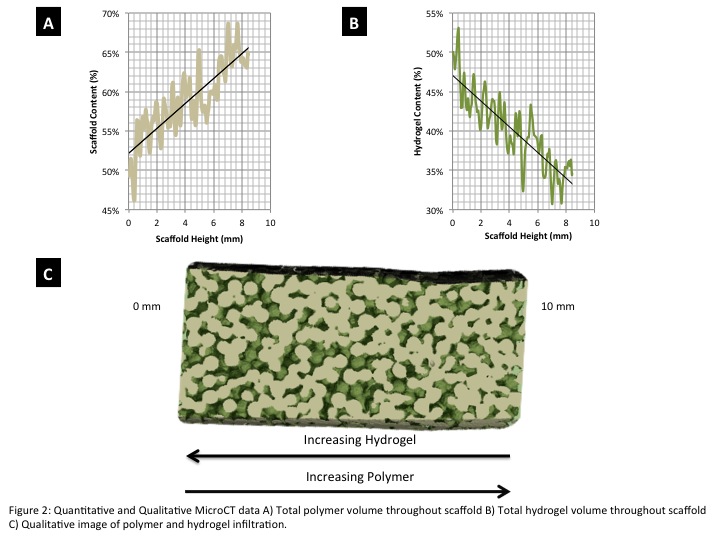
Conclusions: The complexity of OC tissue regeneration lies in the need to not only regenerate cartilage and bone but also the interface region. Based on the quantitative and qualitative expression data, we established a gel matrix suitable for regeneration of the cartilage phase of OC tissue by studying the effects of mechanical strength on cartilage regeneration. With the identified cartilage phase incorporated into the graded PLGA structure we have now designed an inverse gradient matrix system capable of supporting fully integrated OC tissue regeneration.
AO Foundation (S-13-122N); National Science Foundation (1311907); Musculoskeletal Transplant Foundation (MTF); Connecticut Institute for Clinical and Translational Sciences (CICATS)
References:
[1] Csaki C, Schneider PRA, Shakibaei M. 2008. Mesenchymal stem cells as a potential pool for cartilage tissue engineering. Annals of Anatomy-Anatomischer Anzeiger 190
[2] Nukavarapu SP, Dorcemus DL. 2013. Osteochondral tissue engineering: Current strategies and challenges. Biotechnology Advances 31:706-21
[3] Chu CR, Coutts RD, Yoshioka M, Harwood FL, Monosov AZ, Amiel D. 1995. Articular cartilage repair using allogeneic perichondrocyte seeded biodegradable porous polylactic acid (PLA): A tissue-engineering study. Journal of Biomedical Materials Research 29
[4] Schek RM, Taboas JM, Segvich SJ, Hollister SJ, Krebsbach PH. 2004. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Engineering 10
[5] Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. 2011. Continuous Gradients of Material Composition and Growth Factors for Effective Regeneration of the Osteochondral Interface. Tissue Engineering Part A 17
[6] Dorcemus D, Nukavarapu S. Gradient Matrix Design for Osteochondral Tissues Engineering. Proc. Material Research Society Annual Meeting, Boston, MA, 2013:17-23: Cambridge University Press