Polymer topology and graft density affect in vitro mineralisation
-
1
The University of Queensland, School of Chemistry and Molecular Biosciences, Australia
-
2
Universiti Kebangsaan Malaysia, School of Applied Physics, Faculty of Science & Technology, Malaysia
-
3
Queensland Eye Institute, Australia
-
4
Queensland University of Technology, School of Chemistry, Physics and Mechanical Engineering, Australia
Introduction: Many polymeric materials used as implant materials lack functional groups and are therefore considered bioinert. Introducing functional groups onto such otherwise inert polymers is one method that has been explored extensively [1]. In vitro mineralisation in simulated body fluid (SBF) can be used to evaluate a material’s ability to form a strong interface with bone tissue in vivo [2] and early studies demonstrated that such a correlation exists for grafted polyethylene [3]. Fundamental studies on self-assembled monolayers found that the mineralisation capacity in SBF depends on the type functional group (-PO42- > -COO- > -OH) [4]. However, a more complex picture emerges when evaluating the mineralisation capacity of graft copolymers. Our published work on phosphate-containing graft copolymers attached to expanded polytetrafluoroethylene (ePTFE) have illustrated that a linear polymer topology, high retention of phosphate groups and effective coverage of the material surface is required for inducing hydroxyapatite mineralisation [1]. This current study has investigated if similar effects exist for membranes grafted with carboxylate-containing monomers.
Materials and Methods: ePTFE membranes obtained from Pall Corporation were grafted with of acrylic acid (AA) and itaconic acid (IA) [5]-[7]. These grafted materials were characterised by XPS to evaluate carboxylate group density and graft extent (~ surface coverage). Mineralisation was evaluated by immersing the membranes in SBF solution [2] for up to 3 weeks. Samples were examined using SEM/EDX to obtain the morphology and elemental composition of mineral formed. FTIR was used to elucidate the mineral phase formed.
Results and Discussion: The untreated ePTFE membrane did not show any mineralisation after 3 weeks immersion in SBF which correlates with the substrate being highly hydrophobic and lacking functional groups. All substrates with carboxylate-containing graft copolymers induced mineralisation in SBF, however, the extent of mineralisation and the Ca/P ratio of the mineral formed varied. Samples with a low surface coverage resulted in mineral phases of a very high Ca/P ratio of 2.7 [1] while high surface coverage yielded a Ca/P ratio equivalent to Hydroxyapatite as illustrated in the figure.
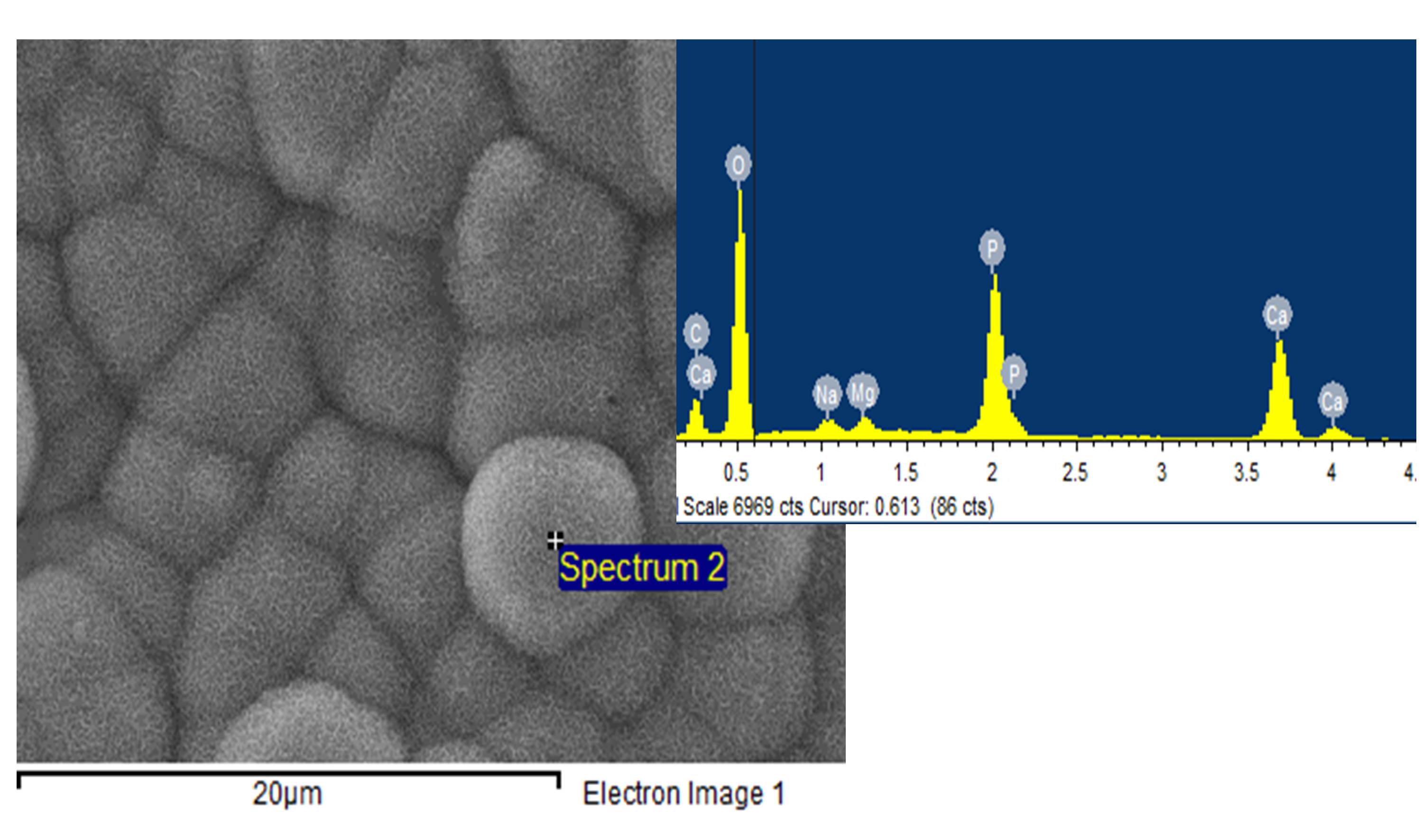
The carboxylate group density also affected mineralisation with high COO/F ratios in the substrate required to effectively induce mineralisation however, there was no benefit observed using AA-co-IA compared to AA graft copolymers. While all these grafted materials have grafted chains of linear topology, a parallel study on crosslinked PAA gels resulted in more acidic mineral phases (eg. Brushite or Monetite) based on EDX.
Conclusion: This work illustrates that high graft density of carboxylate-containing polymers are also capable of promoting hydroxyapatite formation while low graft densities results in other mineral phases. Linear topology is again important for formation of the mineral phase Hydroxyapatite.
N M Hidzir acknowledges the Universiti Kebangsaan Malaysia for a PhD scholarship; This work was carried out in part in the Centre for Microscopy and Microanalysis, The University of Queensland, the Queensland node of the Australian Microscopy and Microanalysis Research Facility (AMMRF)
References:
[1] K Kepa, R Coleman, L Grondahl. In vitro biomineralization of functional polymers. Biosurface and Biotribology doi:10.1016/j.bsbt.2015.09.001
[2] T Kokubo, H Takadama. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27 (2006) 2907
[3] S Kamei, N Tomita, S Tamai, K Kato, Y Ikada. Histologic and mechanical evaluaiton for bone bonding of polymer surfaces grafted with a phosphate-containing polymer. Biomedical Materials Research 37 (1997) 384
[4] M Tanahashi, T Matsuda. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. Biomedical Materials Research 34 (1997) 305-315
[5] N M Hidzir, D J T Hill, D Martin, L Grøndahl. Radiation-induced grafting of acrylic acid onto expanded poly(tetrafluoroethylene) membranes. Polymer 53 (2013) 6063
[6] N M Hidzir, D J T Hill, E Taran, D Martin, L Grøndahl. Argon plasma treatment-induced grafting of acrylic acid onto expanded poly(tetrafluoroethylene) membranes. Polymer 54 (2013) 6536-6543
[7] N M Hidzir, Q Lee, D J T Hill, D Martin, F Rasoul, L Grøndahl. Grafting of Acrylic Acid-co-Itaconic Acid onto ePTFE and Characterisation of Water Uptake by the Graft Copolymers. Applied Polymer Science 132 (2015) 41482
Keywords:
Crystal growth,
surface property,
Polymeric material,
bioactive interface
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Surface and interfacial characterization
Citation:
Grondahl
L,
Mohd Hidzir
N,
Suzuki
S and
Byrne
E
(2016). Polymer topology and graft density affect in vitro mineralisation.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02694
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.