Introduction: Photo-driven reactions have emerged as a versatile tool for biomaterial synthesis because of their ability to construct and functionalize synthetic scaffolds on demand[1],[2]. We report on the synthesis of poly(ethylene glycol) (PEG) hydrogels with tunable mechanical and biochemical properties using a light driven step-growth polymerization. Upon irradiation with light, PEGs functionalized with o-nitrobenzyl alcohols (NB) are converted to aldehyde functionalities, which rapidly react with hydrazine functionalized PEGs to form synthetic scaffolds under cytocompatible conditions (Fig 1). Biochemical signals modified with the NB moiety can be covalently immobilized to the scaffolds with spatial control to impart bioactivity.
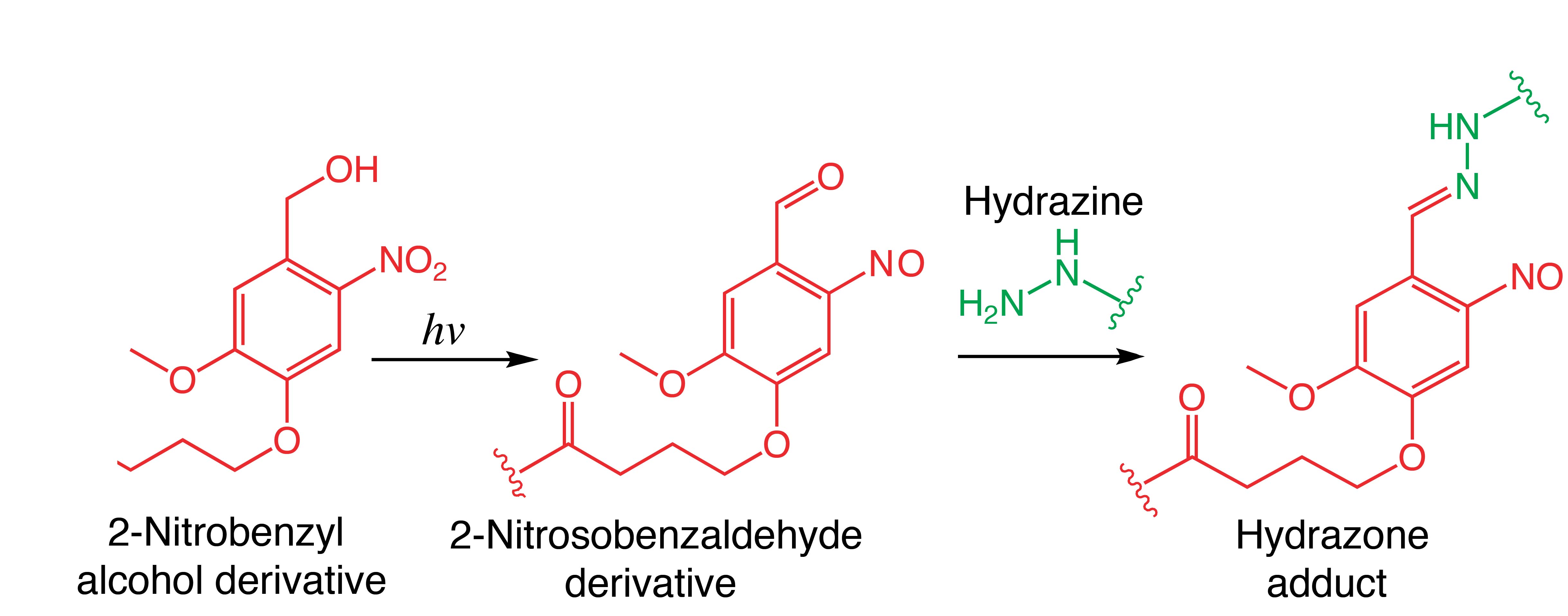
Fig. 1. Reaction between a photo-generated aldehyde and its subsequent condensation with hydrazine to form a hydrazone adduct.
Materials and Methods: 4-arm PEG-hydrazine (PEG-Hy) was synthesized by coupling hydrazinoacetic acid to 4-arm PEG amine. 4-armed PEG-nitrobenzyl-OH (PEG-NB) was obtained by coupling PEG amine to 2-nitrobenzyl-butanoic acid. Hydrogel formation was evaluated via in situ rheological measurements of the shear storage modulus (G’) at 10 rad/s and 1% strain in aqueous conditions at 25°C. Specifically, a 1:1 solution of PEG-Hy and PEG-NB in PBS was exposed to 365 nm light at 5-30 mW/cm2. To demonstrate cytocompatability, human mesenchymal stem cells (hMSCs) were encapsulated in hydrazone hydrogels along with a NB functionalized adhesive RGD peptide. The encapsulated hMSCs were cultured under standard culture conditions for up to 120 h and Live/Dead staining was used to assess cell viability and morphology.
Results and Discussion: Upon exposure to 365nm light, the G’ rapidly increased indicating hydrazone bond formation between PEG-Hy and PEG-NB. Solutions irradiated with higher light intensities (20-30 mW/cm2) reacted more rapidly and had a higher final G’ than those irradiated at lower intensities (5-10 mW/cm2) (Fig 2a). Temporal control of gel properties was demonstrated by shuttering the light for 2 min intervals (Fig 2b). Together, these results quantify the effect of light intensity and exposure time on the evolution of hydrogel mechanical properties.
The viability and morphology of encapsulated hMSCs was quantified using confocal microscopy. Results showed that cells retained a spherical morphology and 78±8% cell survival was noted 120 h post-encapsulation. Since morphology is an important regulator of hMSC differentiation[3], current work focuses on the development of a photogenerated aliphatic hydrazone network with more reversible hydrazone formation kinetics to allow for cell spreading.
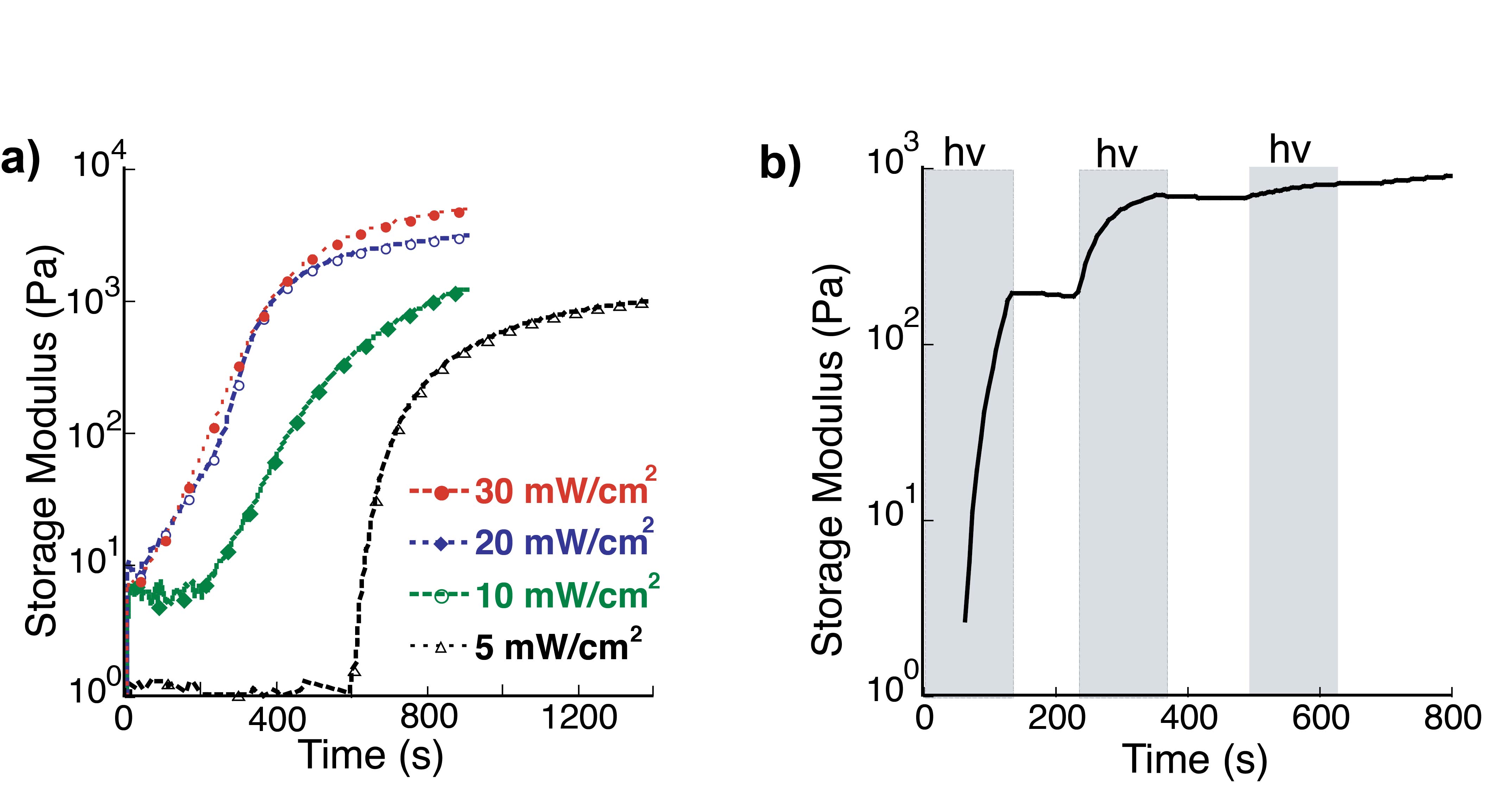
Fig. 2. Effect of light dose on network formation. a) Increasing light intensity influences the generation of the aldehyde functionality, resulting in faster network formation and higher G'. b) Temporal control of the hydrogel evolution is achieved by controlled light exposure. Here, solutions are exposed to 2 min doses of light followed by 2 min in the dark. Polymerization is photocontrolled and only occurs during times of exposure (shaded).
Conclusion: A photocleavage reaction is used to generate a reactive monomer and synthesize hydrazone PEG networks for encapsulating primary cells. Current studies focus on probing the response of hMSCs to in situ regulated mechanical and chemical changes in their microenvironment.
National Science Foundation (Grant NSF-DMR 1408955); Howard Hughes Medical Institute; National Science Foundation Graduate Research Fellowship Program
References:
[1] Tibbitt, M. W., Anseth, K. S. Biotechnol. Bioeng. 2009, 103, 655-663.
[2] Fairbanks, B. D., Schwartz, M. P., Halevi, A. E., Nuttelman, C. R., Bowman, C. N., Anseth, K. S. Adv. Mater. 2009, 21, 5005-5010.
[3] Wang, Y.K., Yu, X., Cohen, D.M., Wozniak, M.A., Yang, M.T., Gao, L., Eyckmans, J., Chen, C.S. Stem Cell Dev. 2011, 21, 1176-1186