Wear-debris induced osteolysis has been recognized as the primary cause of long term total joint replacement (TJR) failure[1]-[3]. To date, it remains unclear what the most effective strategy for treatment of debris induced periprosthetic osteolysis, with no drugs currently approved for use. Potent anti-inflammatory drugs such as anti-TNF-α, IL-1Ra (Anakinra), and methotrexate (MTX) are used to treat rheumatoid arthritis (RA) and show promise for use in treatment of periprothestic osteolysis, but have yet to be comparatively analyzed and thoroughly investigated. Basic questions remain, such as which drug is more effective and what is the most effective delivery method (local or systemic)? We hypothesize that local administration of MTX will reduce UHMWPE induced-inflammatory osteolysis more than systemic MTX, local anti-TNFa or anti-IL1R, due the pleiotropic anti-inflammatory effects of MTX. To test this, we used an established in vivo murine model of particle-induced osteolysis using UHMWPE particles and subsequently evaluated the degree of particle-induced osteolysis between the different regimens that either received local administration of: (1) sham surgery (did not receive UHMWPE nor biologic treatment; negative control), (2) PBS (positive control), (3) anti-mTNF-α, (4) anti-mIL-1R and (5) MTX (local) and compared that to weekly systemic administration of (6) MTX (systemic), where this latter method has been previously demonstrated to reduce particle induced osteolysis[4].
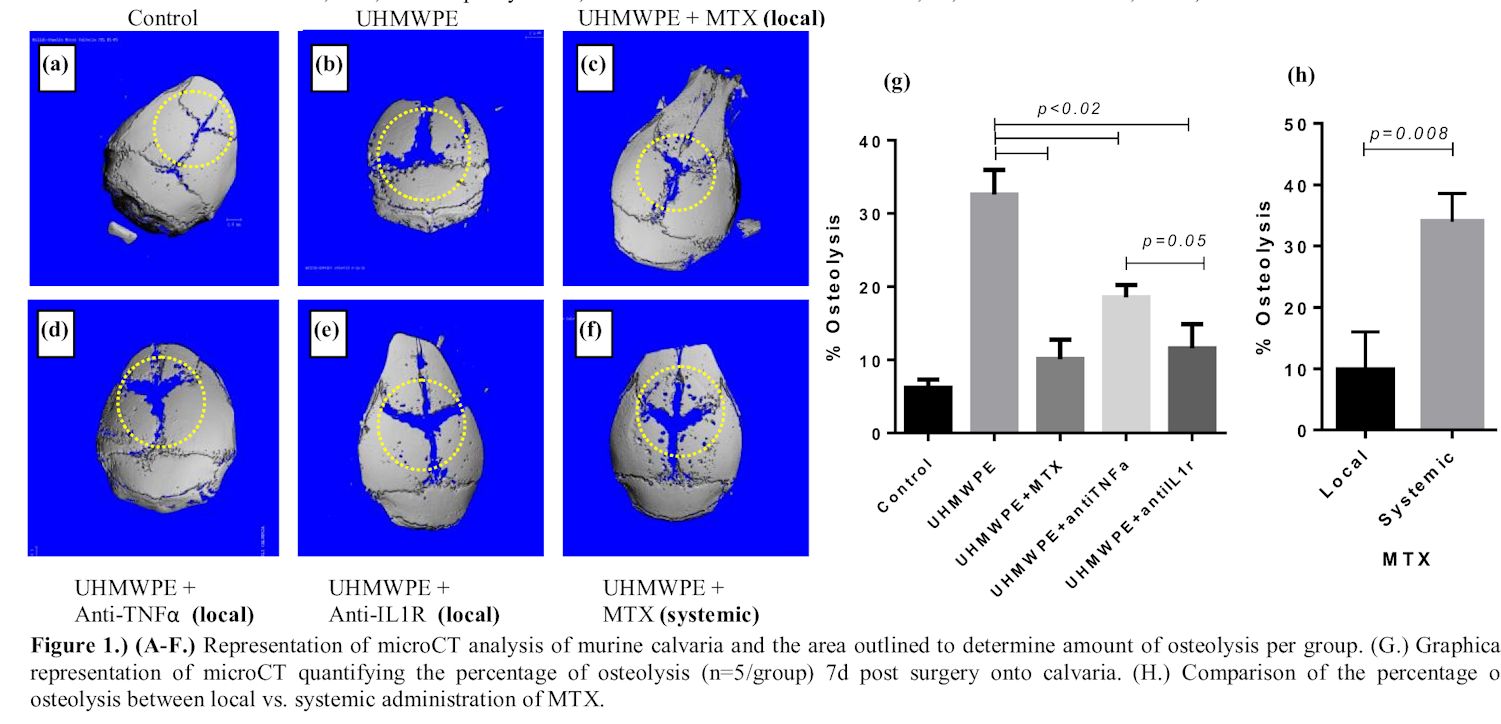
The greatest and most significant (p<0.02) amount of quantifiable osteolysis in the murine model was observed in the positive control group that received UHMWPE particles +PBS, with 32.5% of bone resorption (Fig. 1b). In contrast, local daily administration of either MTX (10.12% Osteolysis) or anti-mIL-1R (11.59% Osteolysis) to murine calvaria that also received UHMWPE particles, did not significantly increase osteolysis compared to the sham-negative control group (6.23% Osteolysis) (Fig. 1c,e,g). While local anti-mTNF-α (18.54%) administration did not significantly decrease the percentage of osteolysis to sham group but was significantly less compared to the UHMWPE particle +PBS treatment, as well as MTX and anti-mIL-1R treatment group at p<0.02 (Fig. 1d,g). However, there was no statistical significance between osteolysis of anti-mTNF-α and anti-mIL-1R group. The local MTX treatment group had significantly less bone resorption though in comparison to anti-mTNF-α (p=0.05) but not to anti-mIL-1R group. Moreover, systemic administration of MTX was not effective, for the amount of osteolysis is similar to UHMWPE group that did not receive any drug agent, and had significantly more bone resorption compared to local MTX treatment (p=0.008; Fig. 1c,f,h).
Our results support our hypothesis that daily local administration of MTX will mitigate UHMWPE-induced inflammatory osteolysis the greatest compared to both local treatment of anti-mTNF-α and anti-mIL-1R or systemic MTX. Local MTX demonstrated to be the most preventive treatment of osteolysis, with bone resorption at only 10.12% and therefore most comparable to that of sham group at 6.23%. More importantly, MTX was more protective given locally as opposed to systemically (p=0.008). Therefore, based on these results, local MTX demonstrates superior promise for use in countering wear-debris induced osteolysis and aseptic loosening.
NIH/NIAMS: AR060782.
References:
[1] Fender D., et al, J Bone Joint Surg Br. 1999;81:577-581.
[2] Callaghan JJ., et al, J Bone Joint Surg Am. 1998;80:704-714.
[3] Kim YH., et al, J Arthroplasty. 1999;14:538-548.
[4] Mediero, A., et al. Arth Rheum, 2015;67:849-855