Pancreatic cancer is one of the most lethal malignancies with a 5 year survival rate of 5% [1]. Gemcitabine (GEM) is currently the first line drug available for treatment of pancreatic cancer. Unfortunately, the clinical application of GEM is severely limited since it can undergo rapid deamination into inactive metabolite [2]. Meanwhile, in the clinical management of pancreatic cancer patients, timely assessment of therapeutic response to a given therapy is critical for making treatment decisions. A new class of fluorescent probes with aggregation-induced emission (AIE) behaviours was reported as a powerful tool in biosensing and bioimaging [3]. In this research, we designed a novel theranostic tetraphenylene (TPE)-containing gemcitabine prodrug TPE-GEM-RGD, which can be used for targeted light-up imaging and selective release of GEM under intracellular reductive environment (Figure 1).
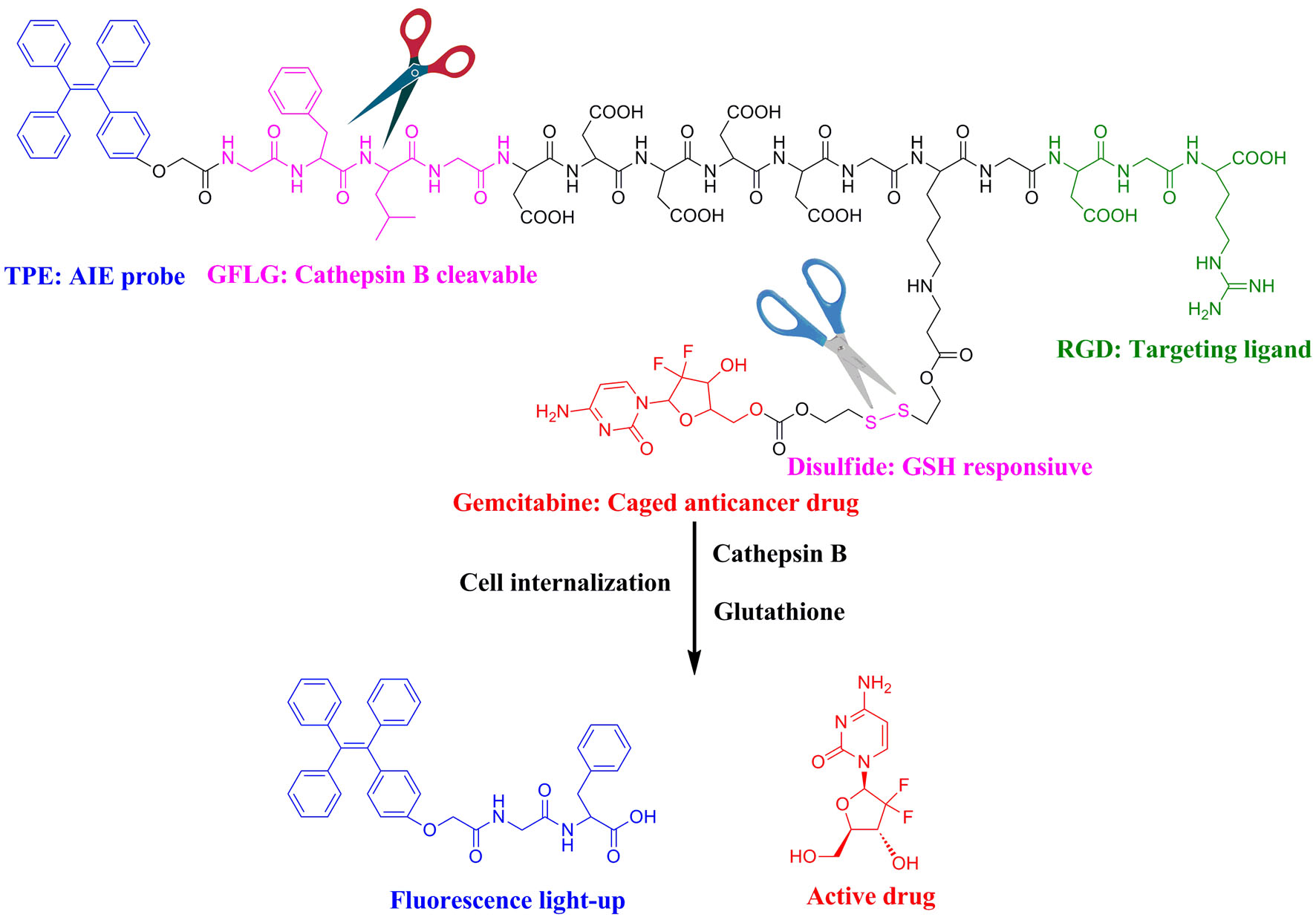
Figure 1. TPE-GEM-RGD for cathepsin B-responsive fluorescence light up and reduction-responsive drug release.
In order to evaluate the light-up capability of TPE-GEM-RGD prodrug, the fluorescent spectra of TPE-GEM-RGD incubated with cathepsin B were recorded. The fluorescence intensity of TPE-GEM-RGD increased steadily with time in the presence of cathepsin B, which might be ascribed to the cleavage of GFLG sequence by cathepsin B.
The reduction-responsive GEM release was studied by incubating TPE-GEM-RGD prodrug with 10 mM of GSH. According to HPLC analysis, upon treating with GSH, GEM was released owing to the appearance of the peak at 4.3 min, which was the characteristic peak of free GEM. Therefore, GEM can be released upon treating with GSH.
Since cathepsin B is overexpressed in pancreatic cancer cells, the capability of TPE-GEM-RGD as cancer cell specific fluorescent light-up probe was studied for live cell imaging [4]. As shown in Figure 2, strong blue fluorescence was observed after BxPC-3 cells were incubated with 50 μM TPE-GEM-RGD. However, when BxPC-3 cells were pretreated with cathepsin B inhibitor CA-074-Me, only very weak blue fluorescence was observed. Moreover, TPE-GEM-RDG prodrug without RGD targeting was used as a negative control. The fluorescent intensity of BxPC-3 cells incubated with TPE-GEM-RDG was much weaker than that incubated with TPE-GEM-RGD. These results demonstrated that the intracellular fluorescence light-up can be achieved.
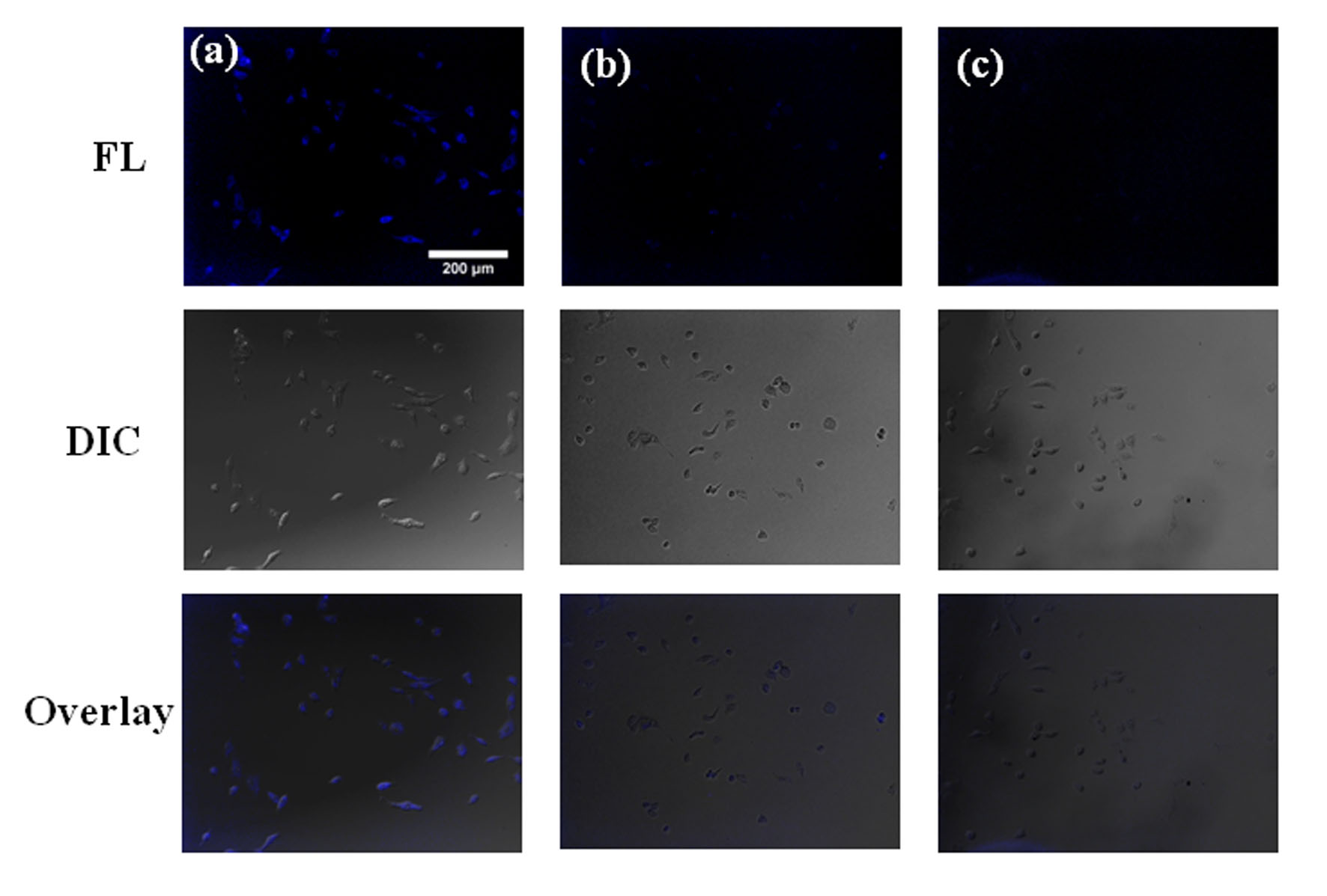
Figure 2. Fluorescent microscopy images of BxPC-3 cells (a) incubated with TPE-GEM-RGD; (b) pretreated with CA-074-Me and incubated with TPE-GEM-RGD; (c) incubated with TPE-GEM-RDG.
In order to identify the reduction-responsive release of GEM, the in vitro cell proliferation inhibition behavior was studied by MTT. The GSH level in BxPC-3 cells can be enhanced or inhibited by pretreating the cells with GSH promoterGSH-OEt or GSH inhibitor BSO [5]. As shown in Figure 3,the BxPC-3 cells pretreated with GSH-OEt or BSO exhibited lowest or highest cell viability, which proved the GSH-responsive behavior of TPE-GEM-RGD prodrug.
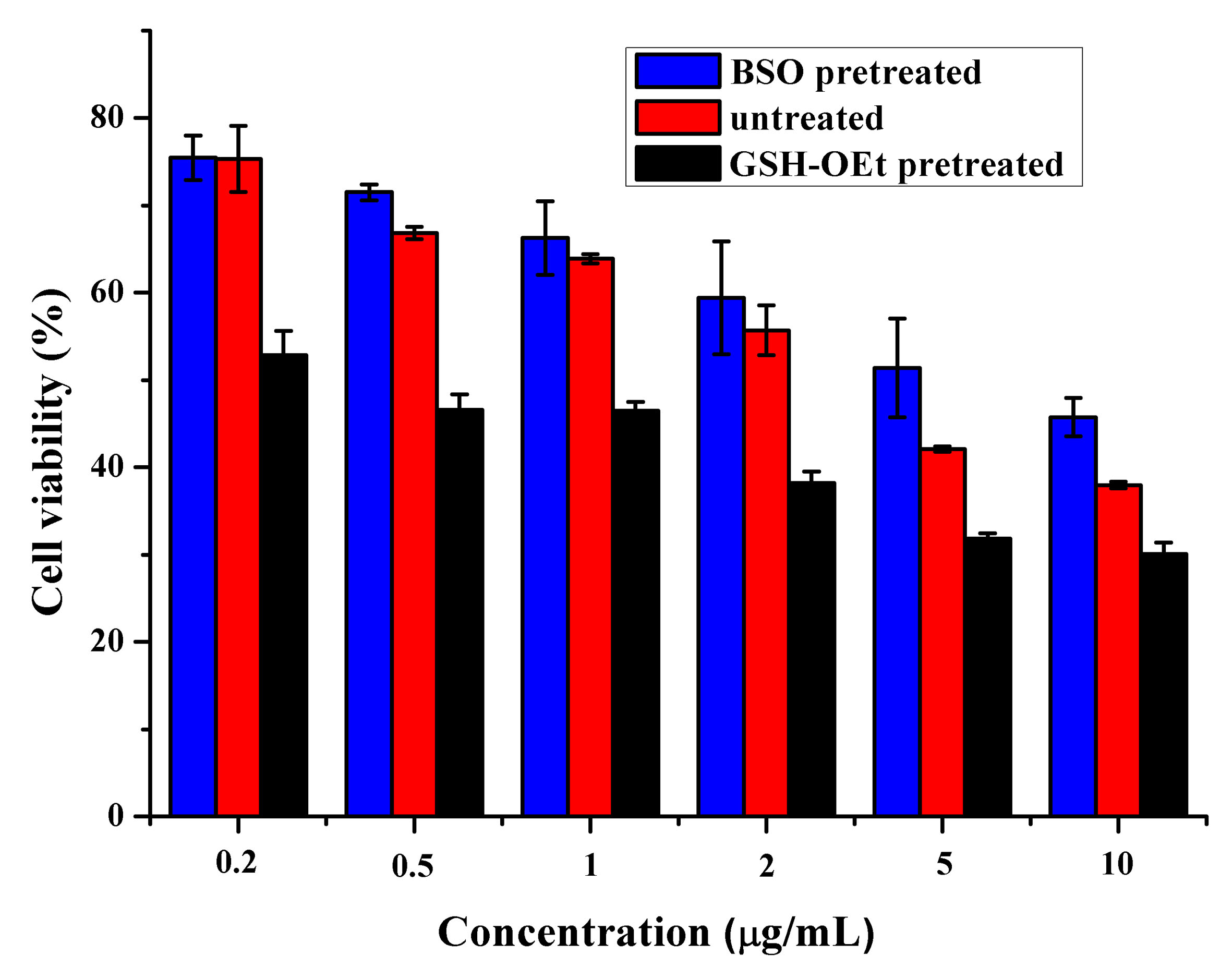
Figure 3. Cell viability of BxPC-3 cells incubated with TPE-GEM-RGD prodrug. BxPC-3 cells were pretreated with BSO or GSH-OEt.
In summary, we have successfully synthesized a theranostic TPE-GEM-RGD prodrug with AIE-based intracellular light-up properties. The TPE-GEM-RGD prodrug was used for targeted light-up imaging and selective inhibition of the proliferation of pancreatic cancer cells.
References:
[1] R. L. Siegel, K. D. Miller and A. Jemal, CA Cancer J. Clin., 2015, 65, 5-29
[2] H. Eda, M. Ura, K. FO, Y. Tanaka, M. Miwa and H. Ishitsuka, Cancer Res., 1998, 58, 1165-1169
[3] J. Liang, B. Z. Tang and B. Liu, Chem. Soc. Rev., 2015, 44, 2798-2811
[4] G. Y. Lee, W. P. Qian, L. Wang, Y. A. Wang, C. A. Staley, M. Satpathy, S. Nie, H. Mao and L. Yang, ACS Nano, 2013, 7, 2078-2089
[5] R. Cheng, F. Feng, F. Meng, C. Deng, J. Feijen and Z. Zhong, J. Control. Release, 2011, 152, 2-12