Introduction: Recent efforts in the design of dynamic 3D cell culture matrices focus on spatially defined real time manipulation of hydrogel structures by single and multiphoton degradation[1]-[3]. As intense focused fs laser pulses can cause cell damage[4], two-photon (2P) degradation in the presence of living cells needs to be an efficient process. Our current research focuses on the design and characterization of a biocompatible hyaluronic acid based hydrogel system incorporating photo-cleavable o-nitrobenzyl (oNB) groups for dynamic cleavage by 2P excitation. The role of the 2P photoinitiator P2CK[5],[6] as photosensitizer for 2P degradation of the hydrogel is investigated.
Materials and Methods: Thiolated hyaluronic acid (HA-SH) was prepared according to the established protocol[7] with the degree of functionalization being 13% per repetition unit. A poly(ethylene glycol) (PEG) photodegradable cross-linker with acrylate terminals was synthesized as previously described[8].
HA-SH was dissolved in serum free medium and neutralized with 0.1 M NaOH to form an 18 mg/mL (6 mM functional groups) stock solution. The PEG-linker was dissolved in PBS giving a stock solution of 150 mg/mL (50 mM functional groups). The solutions were combined in a stoichiometric ratio of functional groups and 40 µL droplets were formed. After gelation (1.5 h) the droplets were swollen either in serum free medium, PBS or solutions of P2CK in PBS with concentrations ranging from 0.01 mM to 1.0 mM respectively overnight at 25°C.
Channels (300 µm x 20 µm x 20 µm) were patterned from the edge into the centre of the gel in medium height with a constant writing speed of 200 mm/s and a varied laser intensity from
5–100 mW using a fs pulsed focused 800 nm laser beam. Afterwards the droplets were swollen in a 5 mg/mL solution of fluorescein isothiocyanate-dextran in PBS before the channels were evaluated by confocal fluorescence microscopy.
Results and Discussion: A hydrogel system was designed to be assembled and later photo-patterned using two orthogonal and biocompatible reactions, Michael addition of thiols to acrylates and radical-mediated cleavage of oNB units respectively. Hyaluronic acid (HA), a major non-sulphated glycosaminoglycan component of extracellular matrix (ECM) was chosen and functionalized with thiol groups to provide the possibility of three-dimensional in situ encapsulation of cells in the hydrogel. The hydrogel was formed by addition of PEG cross-linker containing two photolabile oNB moieties between the PEG chain and acrylate terminals.
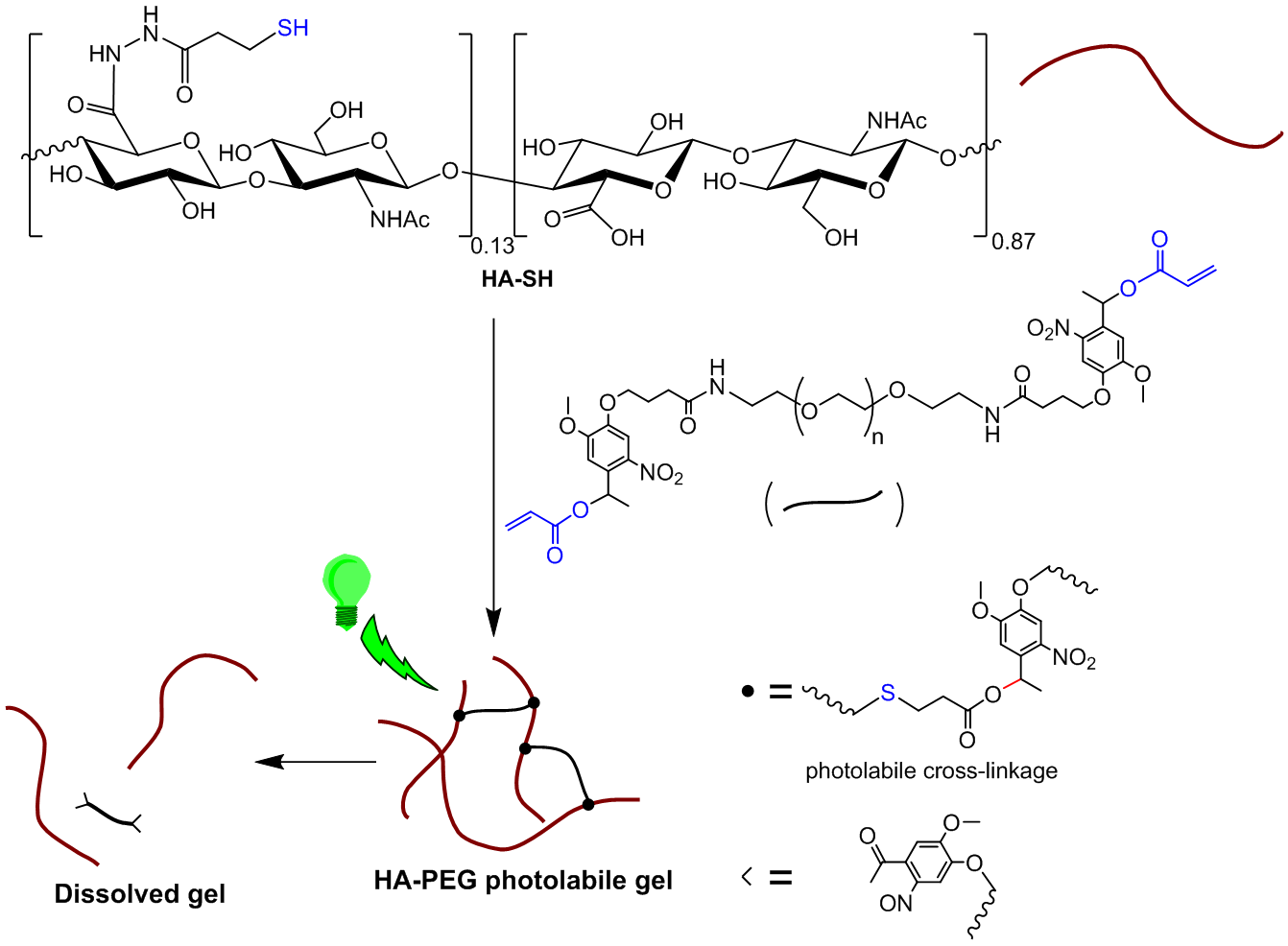
Figure 1: Formation and photo-cleavage of HA-PEG based hydrogel.
After gelation hydrogel droplets were swollen in serum free medium, PBS or, solutions of P2CK at room temperature overnight. Micro-channels were photodegraded into the hydrogels using an 800 nm fs pulsed laser beam with a constant writing speed of 200 mm/s. The laser intensity was varied from 5–100 mW. The experiment demonstrates a significant improvement of 2P photodegradation at low laser intensities in the presence of P2CK.
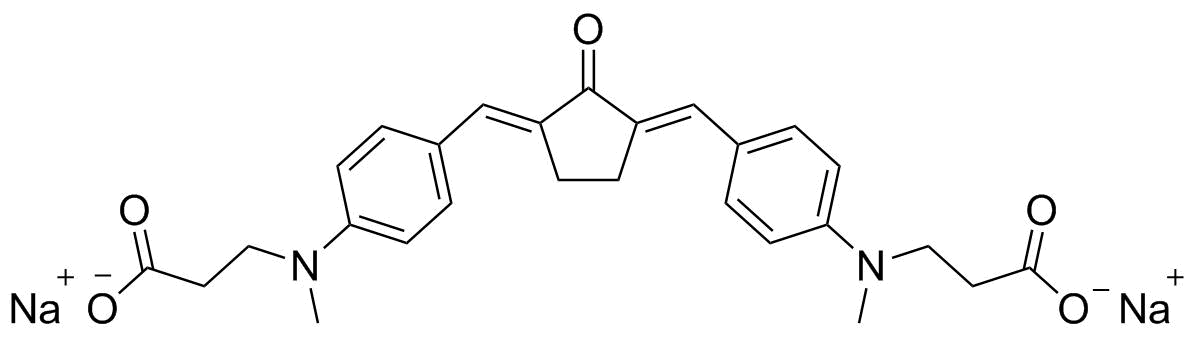
Figure 2: Molecular structure of watersoluble 2P photoinitiator P2CK.
Conclusion: A novel photocleavable hydrogel system was designed and photo-patterned. Significant decrease of the threshold intensity for 2P-degradation by the use of P2CK as photosensitizer was demonstrated. Our preliminary investigations demonstrate the possibility to use this method in the presence of living cells.
The authors would like to acknowledge Johan Verhoeven from Avans Hogeschool (Netherlands) for the synthesis of PEG cross-linker precursors; The authors gratefully acknowledge support from the European Research Council (Starting Grant-307701, A.O.)
References:
[1] DeForest, C. A.; Anseth, K. S., "Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions", Nature Chemistry 2011, 3, 925-931.
[2] Peng, K.; Tomatsu, I.; van den Broek, B.; Cui, C.; Korobko, A. V.; van Noort, J.; Meijer, A. H.; Spaink, H. P.; Kros, A., "Dextran based photodegradable hydrogels formed via a Michael addition", Soft Matter 2011, 7, 4881.
[3] McKinnon, D. D.; Brown, T. E.; Kyburz, K. A.; Kiyotake, E.; Anseth, K. S., "Design and Characterization of a Synthetically Accessible, Photodegradable Hydrogel for User-Directed Formation of Neural Networks", Biomacromolecules 2014, 15, 2808-2816.
[4] Watanabe, W.; Arakawa, N.; Matsunaga, S.; Higashi, T.; Fukui, K.; Isobe, K.; Itoh, K., "Femtosecond laser disruption of subcellular organelles in a living cell", Optics Express 2004, 12, 4203.
[5] Li, Z.; Torgersen, J.; Ajami, A.; Mühleder, S.; Qin, X.; Husinsky, W.; Holnthoner, W.; Ovsianikov, A.; Stampfl, J.; Liska, R., "Initiation efficiency and cytotoxicity of novel water-soluble two-photon photoinitiators for direct 3D microfabrication of hydrogels", RSC Advances 2013, 3, 15939.
[6] Ovsianikov, A.; Mühleder, S.; Torgersen, J.; Li, Z.; Qin, X.-H.; Van Vlierberghe, S.; Dubruel, P.; Holnthoner, W.; Redl, H.; Liska, R.; Stampfl, J., "Laser Photofabrication of Cell-Containing Hydrogel Constructs", Langmuir 2014, 30, 3787-3794.
[7] Shu, X. Z.; Liu, Y.; Luo, Y.; Roberts, M. C.; Prestwich, G. D., "Disulfide Cross-Linked Hyaluronan Hydrogels", Biomacromolecules 2002, 3, 1304-1311.
[8] Kloxin, A. M.; Tibbitt, M. W.; Anseth, K. S., "Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms", Nature Protocols 2010, 5, 1867-1887.