Background: Surgical reconstruction of functional and aesthetic defects, particularly following ablative cancer surgery, is often compromised by limited vascularity and the associated donor site morbidity. A compromised vascular bed is a major obstacle for the successful uptake of a micro-vascular flap.
Aim: Investigate a novel approach of using an injectable scaffolding into a pedicled muscle flap for reconstruction of a critical size mandibular defect.
Materials and Methods: Under general anesthesia bone marrow was aspirated from both the rabbit’s anterior iliac crest. Cells were cultured and expanded according to a validated standard protocol before application in the surgical defect[1]. A critical size bony defect, 20mm x 15mm, was created in the rabbit’s mandibular body[2]. A fixation plate was applied at the inferior border to maintain the mandibular integrity, while the superior border was maintained. A pedicled masseter muscle flap was rotated and adapted to fill the created surgical defect. An injectable calcium sulfate/hydroxyapatite scaffolding material (Cerament Spine Support) loaded with BMP-7 and mesnechymal stem cells was injected into the muscle flap to induce bone formation. Animals were sacrificed at three months following surgery. The quality of bone regeneration was assessed using Cone Beam Computerised Tomography (CBCT), micro CT and histological assessment.
Results: In all the cases sporadic areas of bone regeneration were detected within the muscle flap. In some cases the volume of newly formed bone was more than the volume of bone resected to the create the defect. Bone formation was in close proximity to the injectable scaffold material. Micro CT showed a different trabecular pattern in the regenerated bone to the native bone tissue.
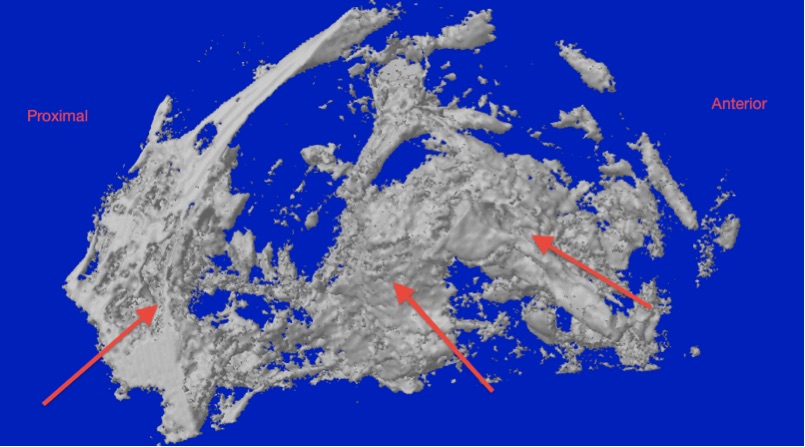
Fig 1 Micro CT showing new bone formed in the defect.
Histologically, the regenerated tissue consisted of a mixture of mature bone with clear Haversion structure and areas of woven bone. Some areas of fibrous tissue “muscle metaplasia” was detected between the newly generated bone and the surrounding muscle fibres. New bone formation was either from the defect edges or
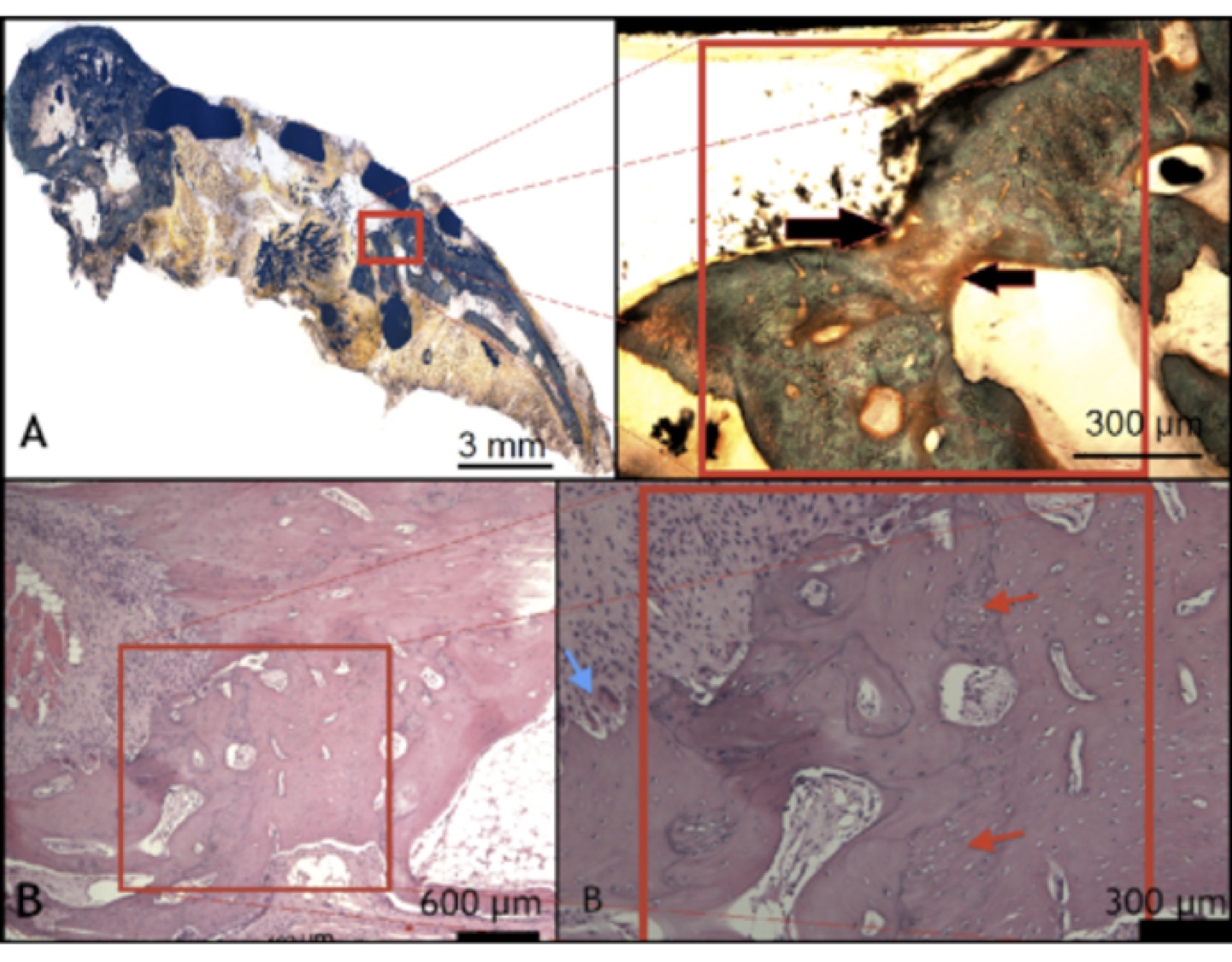
Fig 2 Undecalcified section stained with Goldner’s trichrome, showing resorption of the graft material and new bone formation.
Conclusions: The muscle flap acted as a bioreactor for bone induction under the influence of calcium sulfate/hydroxyapatite, stem cells and BMP-7. Three months is insufficient for complete restoration of the bony defect. The new bone formation concentrated around the areas of the injected cells and material.
Randa Alfotawi was funded by King Saud University, Ministry of Higher Education; Bone Support AB (Lund, Sweden) is thanked for provision of the Cerament™ bone cements
References:
[1] Al Fotawei R, Naudi K, Tanner L, McMahon J, Ayoub Assessment of Cellular Viability on Calcium Sulphate/Hydroxyapatite Injectable Scaffolds. Journal of Tissue Engineering 2013, 204173
[2] Al Fotawei R, Barbenel J, Di Silvio L, Hunter K, McMahon J, Ayoub A. The use of Tri-Calcium Phosphate (TCP) and stem cells for the regeneration of osteoperiosteal critical-size mandibular bony defects, an in vitro and preclinical study. Eu J Cranio-Maxillofac Surg 2014;42:863-869