Statement of Purpose: Hepatocytes are the major parenchymal cells of the liver and play crucial roles in detoxification, metabolism, and protein synthesis. Damages to hepatocytes can lead to hepatic diseases, including hepatitis and cirrhosis and an appropriate in vitro culture system is crucial in understanding the progression as well as the repair of hepatocytes[1]. In this regard, two-dimensional (2D) cell cultures are routinely used to assess hepatocyte viability and functions. However, when cultured on 2D tissue culture plastics (TCP), hepatocytes often exhibit abnormal proliferation and loss of hepatocyte-specific functions[1]-[3]. Additionally, 2D TCP do not provide appropriate cell-extracellular matrix (ECM) interactions and therefore do not allow hepatocytes to establish natural polarity[2]. Three-dimensional (3D) scaffolds recapitulating aspects of the natural ECM microenvironment should provide crucial cell-ECM interactions for hepatocyte culture[3]. Biomimetic thiol-norbornene hydrogels are increasingly used for this endeavor[4]. Here, we present a gelatin-based thiol-norbornene hydrogel system to systematically evaluate the influence of matrix mechanics and biofunctionality on hepatic cell fate in 3D.
Methods: Gelatin-norbornene (GelNB) was synthesized via reacting gelatin with carbic anhydride[5]. Orthogonal thiol-ene photopolymerization (365 nm light, 5mw/cm2, 5 min) was used to crosslink hydrogels from GelNB and poly(ethylene glycol)-tetra-thiol (PEG4SH, 10kDa). Lithium arylphosphinate (LAP) was used as the photoinitiator (1 mM). In selected experiments, heparin-modified GelNB (i.e., GelNB-Hep) was included in the formulations. Hepatocellular carcinoma cells Huh7 were encapsulated in the GelNB hydrogels at 5-million cells per mL. Cell metabolic activity was assessed over time using live/dead staining, Alamarblue® assay, while cellular functions were evaluated by CYP450 activity and urea secretion.
Results: Due to the orthogonal reactivity between norbornene and thiol, the mechanics and bioactivity of gelatin hydrogels can be tuned independently (data not shown). We found that hydrogels with higher content of gelatin (5-7 wt%) with higher stiffness (G’ ~5,500 Pa) supported higher degree of Huh7 cell proliferation (data not shown). The increases in cell metabolic activity over time indicate that GelNB hydrogels were able to support Huh7 growth and proliferation in 3D (Fig. 1A). Interestingly, the inclusion of heparin (in the form of GelNB-hep) in the hydrogel formulation suppressed cell metabolic activity (Fig. 1A) without affecting their viability as evaluated by live/dead staining (Fig. 1B). The incorporation of heparin actually caused less cell death, suggesting a profound impact of immobilized heparin on cell fate processes. Additionally, heparin’s presence also significantly enhanced CYP450 enzymatic activity (Fig. 1C) and urea secretion (Fig. 1D). The enhancement of these hepatocyte-specific functions may be a result of sequestering growth factors from serum through binding to immobilized heparin. Heparin might induce a larger population of the encapsulated cells to enter a state of re-differentiation compared to those encapsulated in GelNB hydrogel.
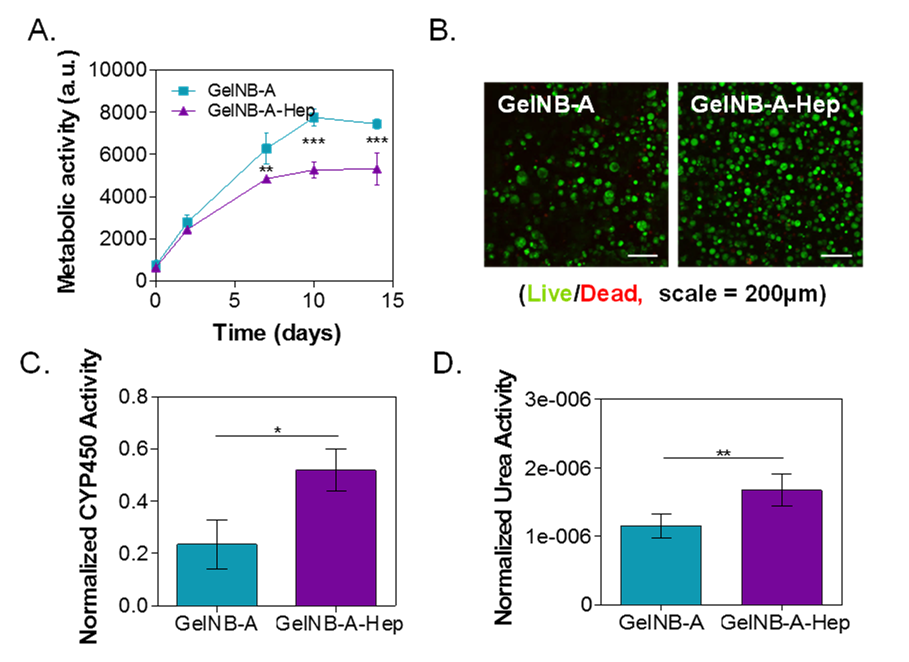
Figure 1. (A) Effect of heparin on metabolic activity of Huh7 cells grown in type A GelNB or GelNB-Hep hydrogels (**p < 0.001; ***p < 0.0001). (B) Representative live/dead staining and confocal Z-stack images of encapsulated Huh7 cells on day 14 post-encapsulation. (C) Normalized CYP3A4 enzymatic activity in Huh7 cells cultured in gelatin-based hydrogels on day 14 (*p < 0.05). (D) Normalized urea secretion of Huh7 cells cultured in gelatin-based hydrogels (**p < 0.001). (n=3, mean ± SD).
Conclusions: In summary, we have prepared modularly crosslinked gelatin-based thiol-ene hydrogels for 3D hepatic cell culture. In addition to the ability to independently tune the gel properties, these new modularly crosslinked GelNB-Hep hydrogels show high potential in answering questions related to hepatic cell fate in 3D. This material system should be of great interests in regenerative medicine and hepatic tissue engineering.
References:
[1] Lau TT, Lee LQ, Leong W, Wang DA. Biomed. Mater. 2012;7:065003.
[2] Malinen MM, Kanninen LK, Corlu A, Isoniemi HM, Lou YR, Yliperttula ML, et al. Biomaterials 2014;35:5110-21
[3] Lin TY, Ki CS, Lin CC. Biomaterials 2014;35:6898-906.
[4] Lin CC. J Appl. Polym. Sci. 2015;132:41563.
[5] Munoz Z, Shih H, and Lin CC. Biomater. Sci. 2014;2:1063-1072.