Introduction: Biological tissues/organs consist of hierarchical organization of various extracellular matrix (ECM) molecules with spatial arrangement of cells and soluble factors. To generate the functional tissue equivalents in vitro, engineered scaffolds should mimic the structural, mechano-chemical, and cellular complexity. Various combinations of biochemical and biomaterial strategies have been attempted to engineer such environment, however, most of them end up restoring a chaotic environment. Adopting a structure-centric biomimetic approach, we have developed highly aligned fibrous hydrogels using microfluidics that show nano- to micro- to macroscale hierarchy of collagen. These hydrogels showed powerful bioactivity to sequester inorganic ions from simulated body fluid (SBF) and guide biomineralization in a manner similar to collagen. Moreover, smart fabrication method allowed us to broaden the scope of these hydrogels to achieve self-assembled nanocomposites with the use of graphene. Interestingly, these graphene nanocomposites not only helped to achieve mechanical properties near native skeletal muscle tissue, but it also influenced the differentiation of myoblasts into myotubes by providing conductive nanoplatforms. Moreover, simple electrostatic interaction allows us to store small molecule and peptide in the scaffolds for their controlled local release.
Methods: Natural polysaccharides were used for preparing three different types of aligned fibrous hydrogel scaffolds. A custom designed microfluidic chamber was used for their self-assembly into fibrous hydrogel scaffolds. Hierarchical structure at different scale lengths (nm to cm) of fabricated hydrogels was investigated using light and electron microscopy. These fibrous hydrogels were further incubated in SBF at 37⁰C for three days to study their mineralization potential in vitro. Chemical and crystalline structure, alignment and spatial distribution (inter/intrafibrillar) deposition of deposited minerals were investigated using fourier transform infra-red spectroscopy (FTIR), X-ray diffraction (XRD), scanning and transmission electron microscopy (SEM, TEM). Further, graphene nanocomposites were fabricated using the same microfluidic method. Mechanical properties were characterized using eXpert 7601 Table Top Universal Testing System from ADMET, following by SEM imaging of the fractured scaffolds. Cytocompatibility was assessed using alamarBlue® assay. Differentiation was studied after 14 days culturing of myoblasts on the scaffolds. FITC and FITC-BSA were used as model moieties for drug and peptide, respectively, to assess the potential of fibrous hydrogel scaffolds for controlled local release.
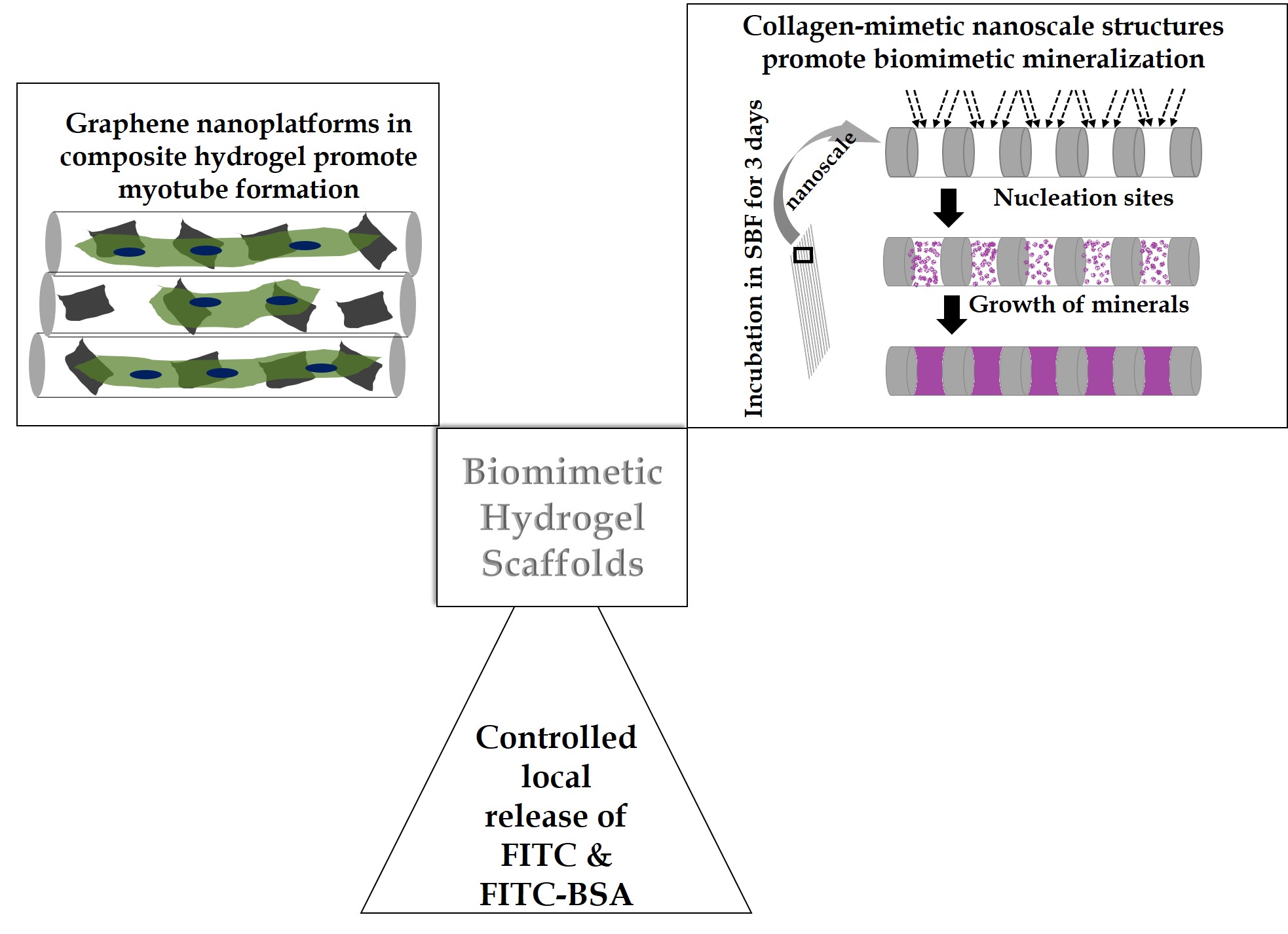
Results and Discussion: Self-assembled scaffolds showed multi-scale hierarchy and characteristic light-dark bands at nanoscale as shown in schematic in figure 1. Moreover, three day of incubation in SBF resulted in patterned deposition of chemically biomimetic minerals on the scaffolds as well as inside the scaffold. This demonstrates powerful bioactivity resulting from steric influence of nanoscale collagen-mimetic features and multiscale hierarchy. Biomimetic chemical nature of the deposited minerals complements the structure-activity relationship. Moreover, patterned mineral deposition on the scaffold and inside the scaffold points to important role of hydrogels similar to collagen. Graphene nanocomposite hydrogel scaffolds showed uniform distribution of the immobilized graphene sheets all over the scaffolds. Nanocomposites improved mechanical properties of fibrous scaffolds significantly. Interestingly, myoblasts differentiated into multinucleated myotubes after 14 days of culture. This demonstrates the synergistic influence of mechanotransduction and underlying conductive graphene platforms on the myoblast by instructing them to differentiate. Additionally, FITC and FITC-BSA release profile showed their release for nearly a week, suggesting endless possibilities for incorporation of various drugs and peptides for controlling regeneration.
Conclusion: The biomimetic fibrous hydrogel scaffolds provide versatile bioactive platform for various tissue engineering applications.