Introduction: Diabetes is one of the most common chronic diseases caused by abnormally insufficient secretion of insulin. Currently, multiple, subcutaneous self-injections are generally accepted for insulin treatments. However, it commonly causes poor patient compliance due to pain and inconvenience. To resolve this, we suggest a battery-less implantable infusion pump for on-demand, pulsatile delivery of insulin [1], the whole surface of which was also coated with Parylene C for implantation. In this study, we performed in vitro drug release test, which exhibited that the pump could release an accurate amount of insulin only at the time when a magnetic field was applied from the outside of the body. The in vivo efficacy tests with STZ-induced diabetic rats demonstrated that the pump could effectively lower the blood glucose level reproducibly. Now, the work is in progress to assess the biocompatibility by performing histological analysis on the tissues around the implanted pump.
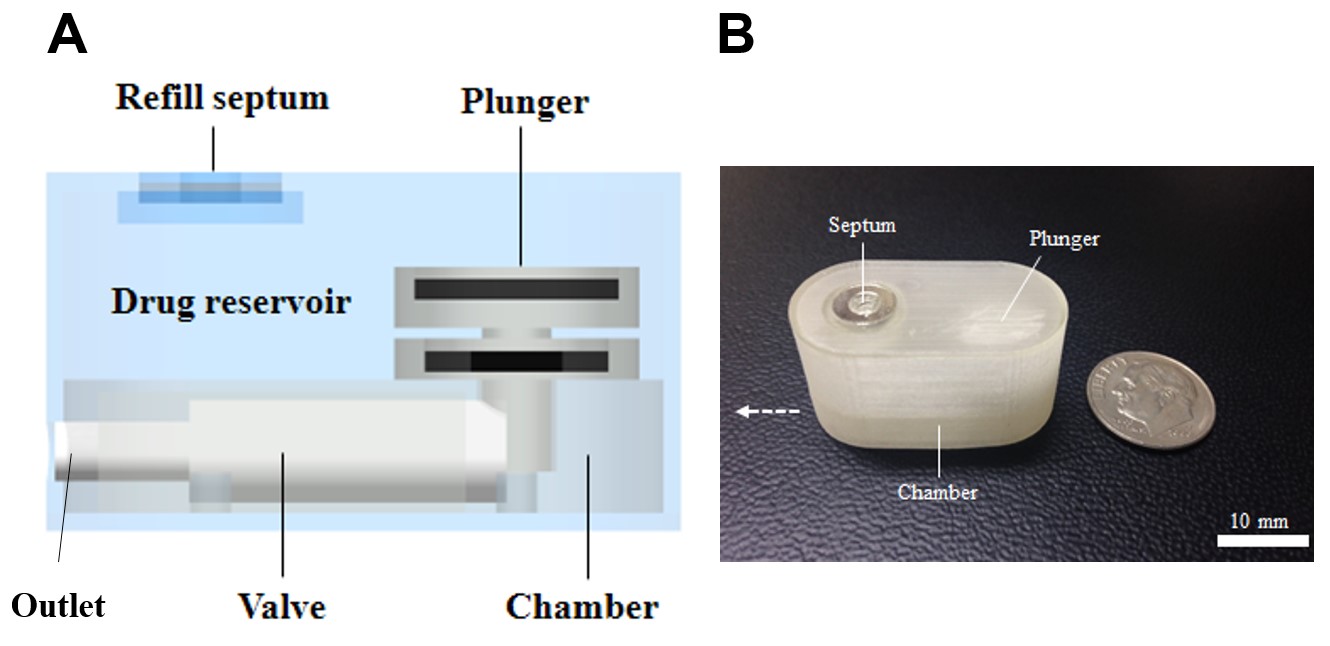
Methods: The pump consists of three distinct units: 1) drug reservoir, 2) plunger and 3) chamber (Figure. 1). The drug reservoir was filled with an insulin solution (5 mg/ml). Thus, when an external magnetic field was applied, a plunger moved upward to suck insulin from the reservoir into the chamber. When a magnetic field was removed, a plunger moved downward to infuse insulin to the outside of the pump. We also coated the whole surface of the pump with parylene C, considering its biocompatibility after implantation. With this pump, we performed in vitro drug release experiments in phosphate buffered saline (PBS; pH 7.4) at 37oC. For in vivo tests, the pump was subcutaneously implanted in diabetic animal models (rats) and actuated via magnetic field applied from the outside of the body.
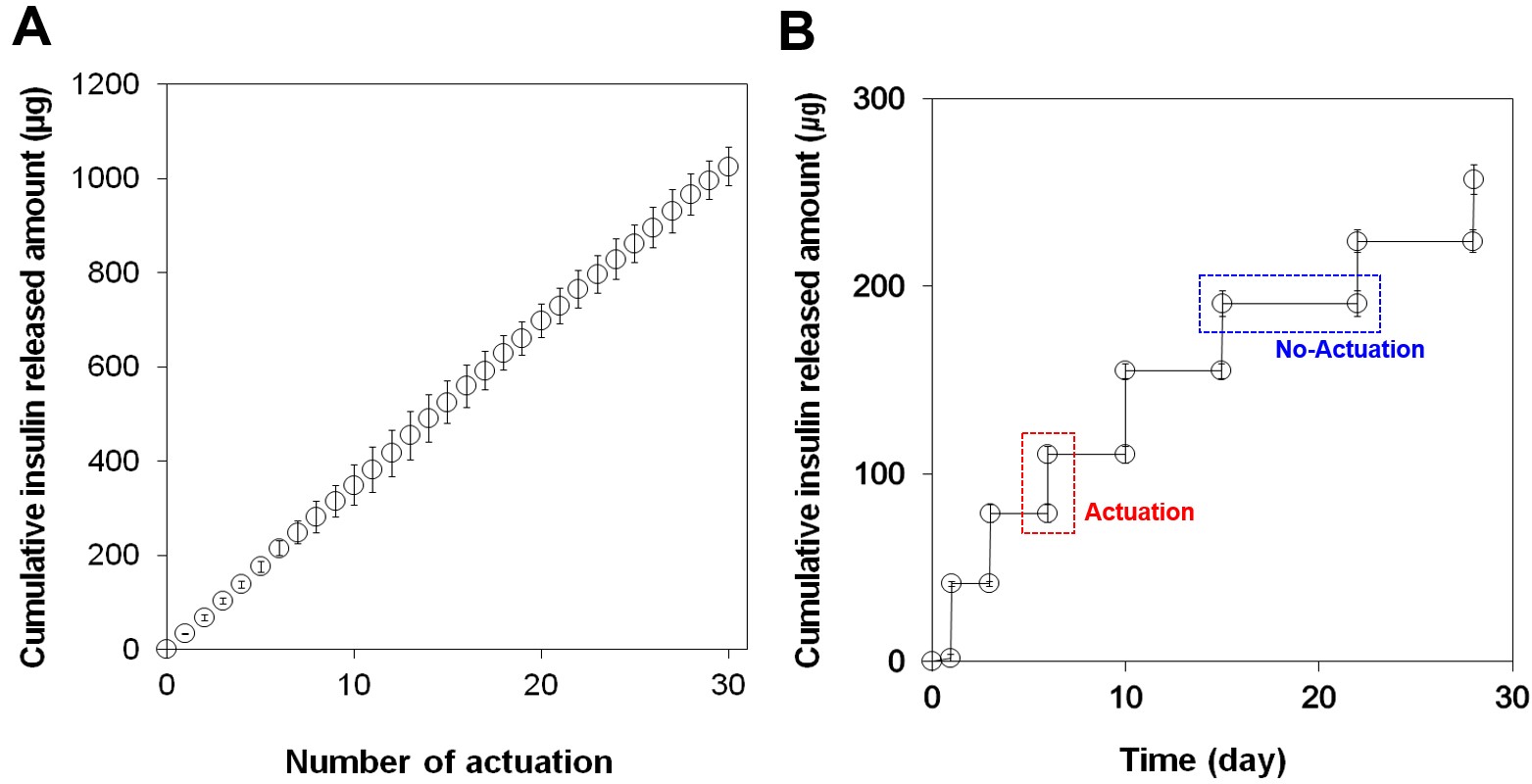
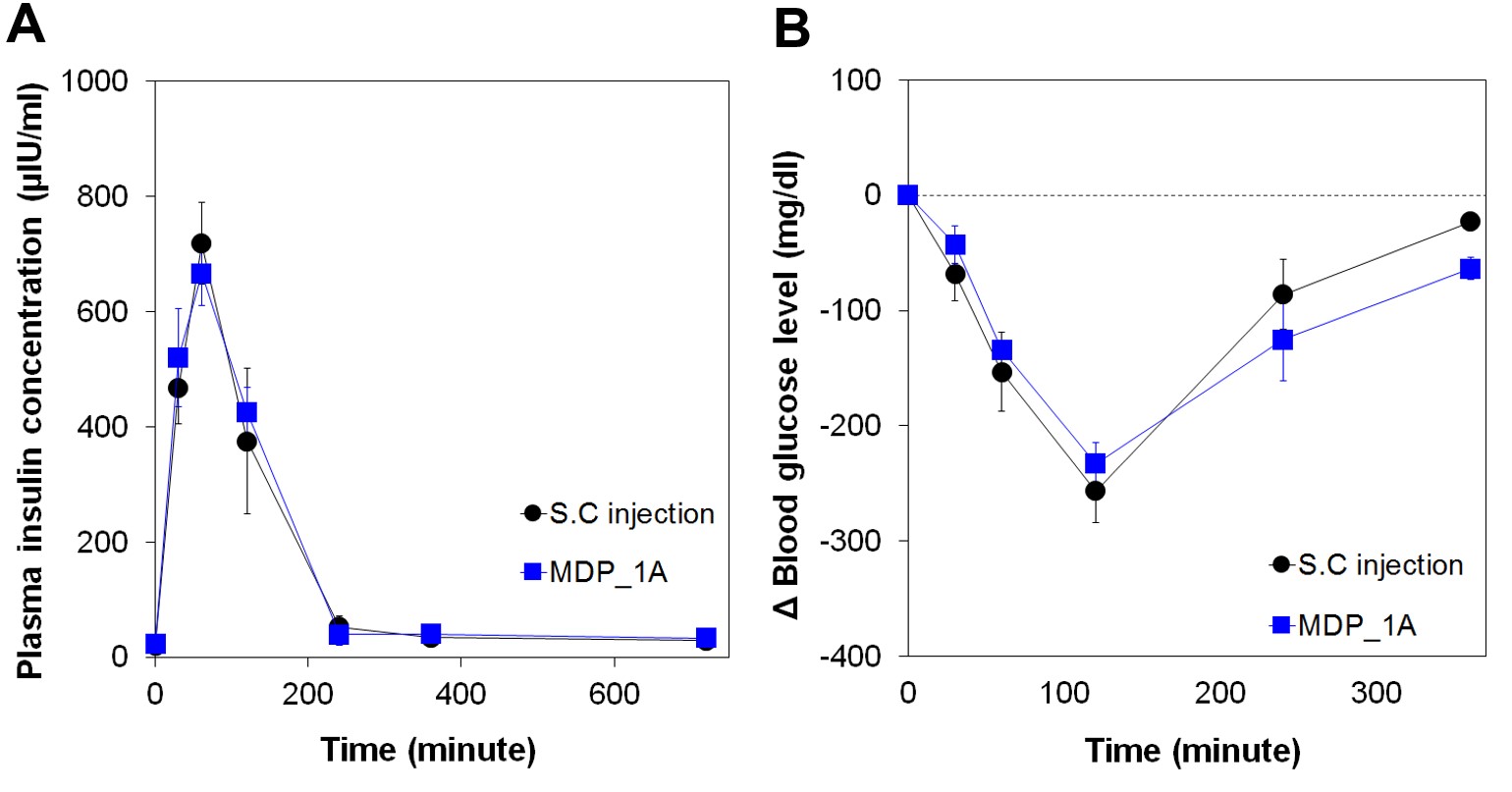
Results and Discussion: According to the in vitro experiments (Figure. 2), an accurate amount of insulin could be released only at the time when an external magnetic field was applied, which could be reproducible for 28 days. The in vivo PK results revealed that the pump indeed exposed insulin in plasma, where its maximum concentration was found at 60 min (Figure.3). Notably, this PK profile was almost same with that of the group treated with subcutaneous injection of the same dose insulin (SC group). The PD profiles were evaluated by measuring the decrease in glucose concentration in plasma. The results indicated that the PD profile of the pump was again almost same with that of the SC group, showing the maximum drop at 120 min after insulin administration.
Conclusions: In this work, we suggest a battery-less, implantable infusion pump for on-demand, pulsatile delivery of insulin which was shown effectively operative in both in vitro and in STZ-induced diabetic rats. Therefore, we conclude that implantable infusion pump has a high potential for on-demand, pulsatile drug release for the treatment of diabetes.
Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (HI14C2194); BK21 Plus Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education (grant numbers 22A20130011025)
References:
[1] Pickup, J.C. et al., NAT REV ENDOCRINOL 8, 425-433 (2012).