Introduction: Bone fractures are quite common and while most heal naturally, severe bone injuries such as those caused by trauma often do not heal on their own and require a tissue graft for repair. Many of these grafts fail due to a compromised blood supply. These failures suggest the rate and extent of vessel in-growth are crucial and needed for successful bone regeneration[1],[2]. Other studies have also indicated that biomimetic extracellular matrix components such as hydroxyapatite (HA) and collagen are ideal as they are biocompatible and occur naturally in bone[3]. As such, spatial-temporal seeding variations of co-culture cells in 3D HA collagen composite scaffolds were evaluated in this study for enhancement of osteogenesis and angiogenesis.
Materials and Methods: Composite scaffolds were prepared by casting 4 mg/ml collagen hydrogels within a porous 3D HA scaffold. Using a previously described template coating process[4], HA scaffolds were prepared with an average porosity of 80%. Initial experiments demonstrated an increase in VEGF production on day 7 when bone mesenchymal stem cells were seeded alone on composite scaffolds. In this study, optimized concentrations of human embryonic palatal mesenchymal cells (HEPMs) and human umbilical vein endothelial cells (HUVECs) were seeded with spatial-temporal variation (Fig. 1): Group 1 having HEPMs seeded 7 days before HUVECs seeding, Groups 2 having HEPMs seeded 6 hours before HUVECs seeding, and Group 3 having HEPMs and HUVECs cast in the composite scaffolds. Production of vascular markers (VEGF, Ang-1, and angiogenin) and alkaline phosphatase (ALP), an early osteogenic marker, were measured at regular intervals using ELISA. Groups were compared using 2-way ANOVA across time and Tukey’s test (at p<0.05).
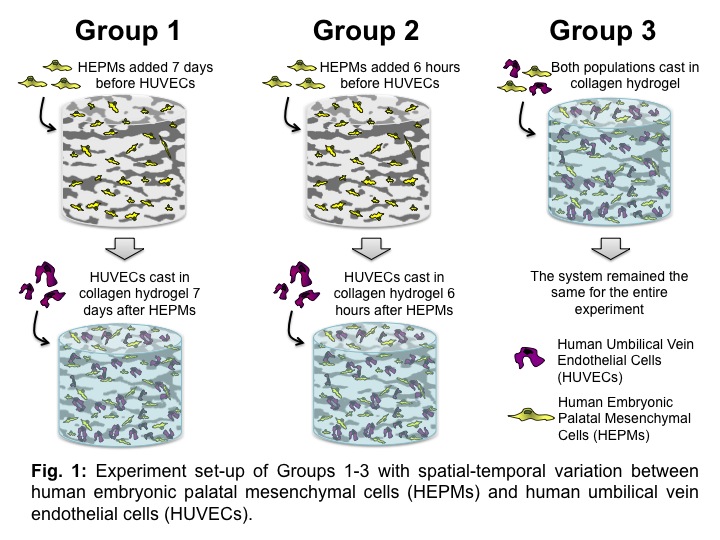
Results and Discussion: Groups 1 and 3 showed similar trends in ALP and vascular markers throughout the duration of the experiment (Fig 2a and b). Group 1 and 3 had an initial peak of ALP, which is indicative of osteoblast differentiation since ALP is an early osteogenic marker (Fig 2a). However, Group 2 had reduced and fairly consistent ALP production when compared to Groups 1 and 3 throughout the experiment, suggesting delayed HEPM attachment on the HA scaffold and hydrogel. All Groups showed an initial early increase in vascular markers Ang-1 (essential for organization, integrity, and maturation of neo-vasculature[5]) and angiogenin (potent inducer of neovascularization in vitro[6]) (Fig 2c and d). However, vascular marker maturation levels were observed to decrease over time, suggesting the entrapment of vascular proteins in newly formed ECM. Hence, results imply both bone and vascularization are occurring.
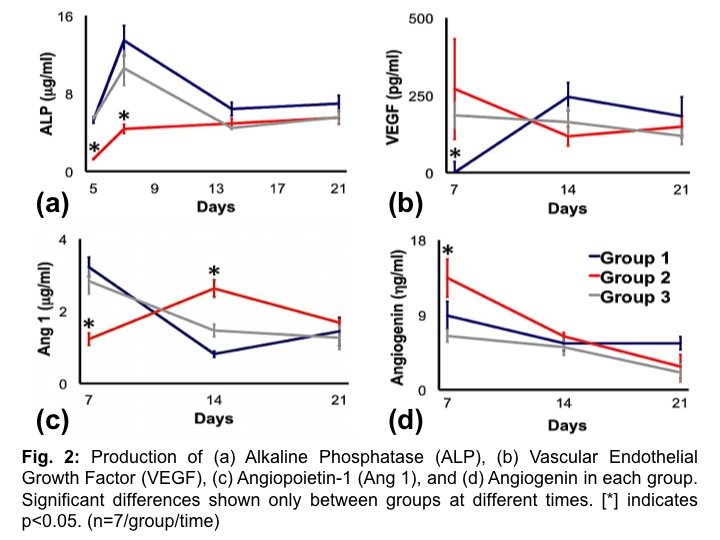
Conclusions: Osteogenic and angiogenic differentiation are indicated for all scaffolds, suggesting the occurrence of bone and vascular activities. However, spatio-temporal differences in cell seeding resulted in profound differences early at day 7 which might impact tissue organization and maturation.
NIH/NIGMS MARC U*STAR GM007717; UTSA College of Engineering
References:
[1] Shah, A. R., S. R. Shah, S. Oh, J. L. Ong, J. C. Wenke, and C. Mauli Agrawal. "Migration of Co-cultured Endothelial Cells and Osteoblasts in Composite Hydroxyapatite/Polylactic Acid Scaffolds." Annals of Biomedical Engineering Ann Biomed Eng 39.10 (2011): 2501-509.
[2] Novosel, E. C., C. Kleinhans, and P. J. Kluger. "Vascularization Is the Key Challenge in Tissue Engineering." Advanced Drug Delivery Reviews 63.4-5 (2011): 300-11.
[3] Bose, S., G. Fielding, S. Tarafder, and A. Bandyopadhyay. “Trace Element Doping in Calcium Phosphate Ceramics to Understand Osteogenesis and Angiogenesis.” Trends in biotechnology 31.10 (2013).
[4] Appleford, M. R., S. Oh, N. Oh, and J. L. Ong. "In Vivo Study on Hydroxyapatite Scaffolds with Trabecular Architecture for Bone Repair." Journal of Biomedical Materials Research Part A J. Biomed. Mater. Res. 89A.4 (2009): 1019-027.
[5] Brindle, N. P.j., P. Saharinen, and K. Alitalo. "Signaling and Functions of Angiopoietin-1 in Vascular Protection." Circulation Research 98.8 (2006): 1014-023.
[6] Pavlov, N., J. Frendo, J. Guibourdenche, S. A. Degrelle, D. Evain-Brion, and J. Badet. "Angiogenin Expression during Early Human Placental Development; Association with Blood Vessel Formation." BioMed Research International 2014 (2014): 1-17.