Introduction: The ideal scaffold for bone regeneration needs a number of requirements, such as biostability until the formation of mature tissue, high porosity for cell migration, extracellular matrix (ECM) deposition and vascularization, and non-immunogenicity. Moreover it should be bioresorbable, osteoconductive and possibly osteoinductive. Processed bovine spongy bone xenografts, coated with poly(L-lactic-co-ε-caprolactone) (PLCL) and added with gelatin are commercially available as a new class III medical device (SmartBone®, IBI S/A, Switzerland) [1]. This study was aimed at investigating the process of new bone formation in patients treated with SmartBone® for sinus lift procedures prior to dental implants.
Materials and Methods: Tissue biopsies deriving from screw removal in 5 patients treated with granular SmartBone® for sinus lift were collected at different times after implantation (4-9 month range), formalin-fixed, decalcified in 10% EDTA pH 7.4 for 15 days at 4°C, and processed for histochemistry to assess: cell/tissue morphology (H&E), glycoproteins (PAS staining), glycosaminoglycans (Alcian Blue pH 2.5 staining), collagen (Van Gieson); and immunohistochemistry to assess bone markers (fibronectin, collagen type I, osteocalcin and TGFβ1). Histomorphometric analysis and bone-particle conductivity index (BPCi) were calculated on 6 histologic sections at 20× magnification using ImageJ software.
BPCi = ∑ lenghts of new bone in contact with SmartBone® particles/∑ perimeters of SmartBone® particles
Results and Discussion: Biopsies collected at 4 months post-implant showed the contemporaneous presence of SmartBone® (21.00%±0.08%), new bone (24.55%±0.07%) and relevant amount of fibrous tissue with formation of new vessels (54.44%±0.09%). BPCi was 0.34±0.14, indicating SmartBone® as a very good scaffold for new bone formation. Bone ECM molecules were highly expressed in the newly formed bone and osteoblasts were visible on SmartBone® surfaces (Figure 1).
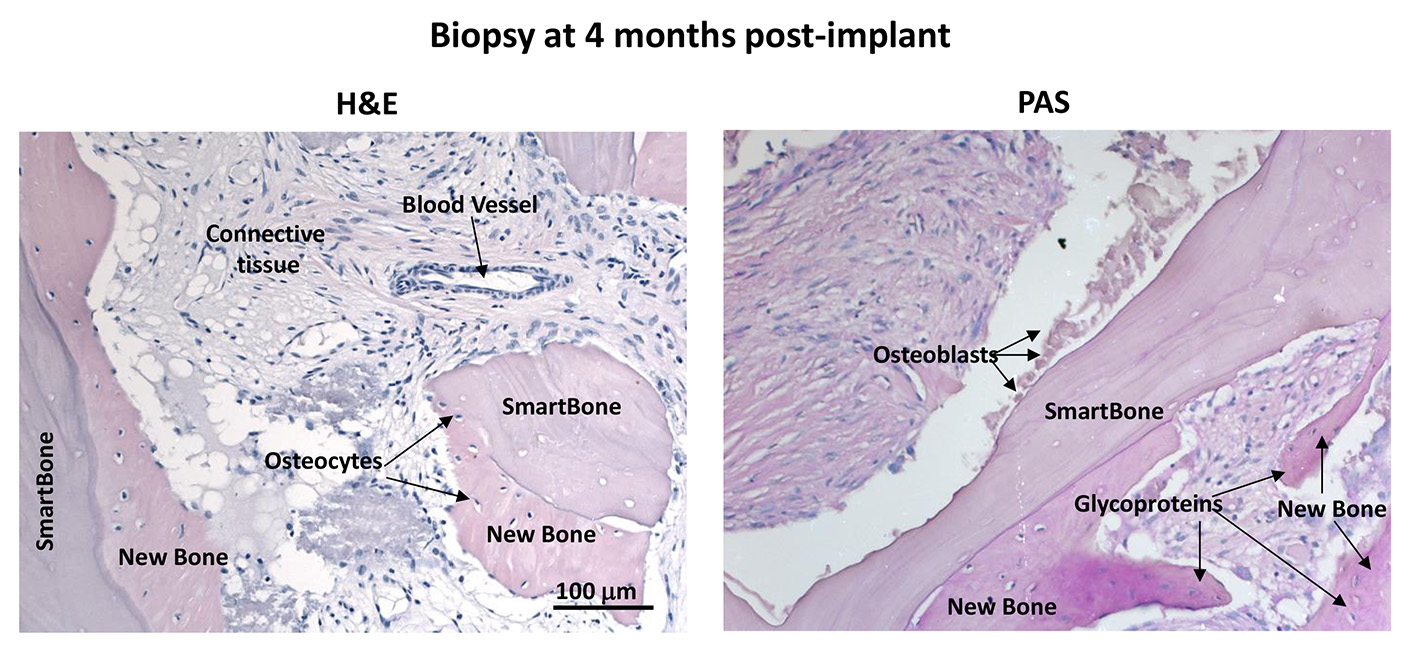
Biopsies collected at 6 months post-implant showed large areas of new bone, while SmartBone® was rarely present. Such extensive resorption and substitution of SmartBone® prevented longer-term BPCi evaluation. The process of new bone formation was corroborated by the high density of osteocytes and the presence of osteoblasts close to bone growth frontlines, while the process of SmartBone® resorption was supported by the detection of some osteoclasts. Biopsies collected between 7-9 months post-implant revealed allover new bone, with well-defined bone scars and oriented bone lamellae, thus suggesting almost complete resorption of SmartBone® and its replacement with mature bone (Figure 2). No inflammatory cells were detected.
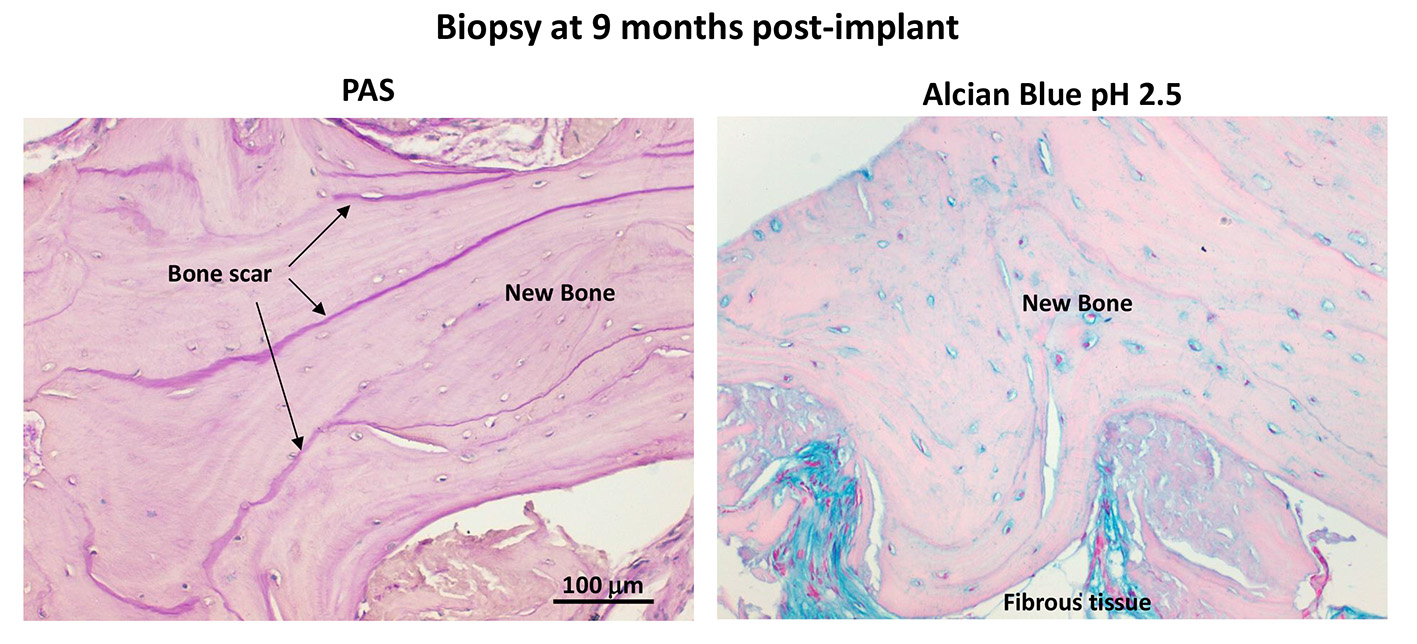
Conclusions: These results indicate that SmartBone® was able to promote a gradual process of new bone formation. The absence of inflammatory infiltrates and the presence of a complete mature bone at 7-8 months from the implant suggest that SmartBone® is a good scaffold for bone regeneration surgery.
References:
[1] G. Pertici, F. Carinci, G. Carusi, D. Epistatus, T. Villa, F. Crivelli, F. Rossi, G. Perale. Composite polymer-coated mineral scaffolds for bone regeneration: from material characterization to human studies. Journal of Biological Regulators & Homeostatic Agents (2015) [in press].