Introduction: By using 3D printing techniques such as fused deposition modeling (FDM) and selective laser sintering, tissue engineering scaffolds with desirable properties can be produced[1],[2]. Conventional FDM requires heating a polymer wire to form molten “ink”, which damages biomolecules if they are incorporated in the “ink” during scaffold fabrication. Therefore, developing new techniques based on 3D printing for delivering biomolecules is very important. For bone tissue engineering, delivering both bone morphogenetic protein-2 (BMP-2) and calcium phosphate (Ca-P) could provide scaffolds with osteoinductivity and osteoconductivity[3]. In this study, a modified commercial desktop 3D printer was used to fabricate rhBMP-2 and Ca-P nanoparticle incorporated poly(L,lactic acid) (PLLA) scaffolds.
Materials and Methods: A commercial 3D printer (Replicator® 2X, Makerbot®, USA) was modified for scaffold fabrication[4]. By using w/o polymer emulsions as the “ink” (containing rhBMP-2 or Ca-P nanoparticles) and refrigerating the printing stage to -40°C, 3D scaffolds were printed and the as-printed patterns solidified immediately, which made 3D printing continuous. 3D printing was followed by freeze-drying and washing to remove solvent. SEM was used to examine scaffolds. Compression tests were conducted for determining scaffold properties. In vitro release of rhBMP-2 and Ca2+ ions were investigated through 28-day immersion experiments. Live and dead assay and MTT assay were used to study the viability of human bone marrow derived MSCs (hBMSCs) on scaffolds.
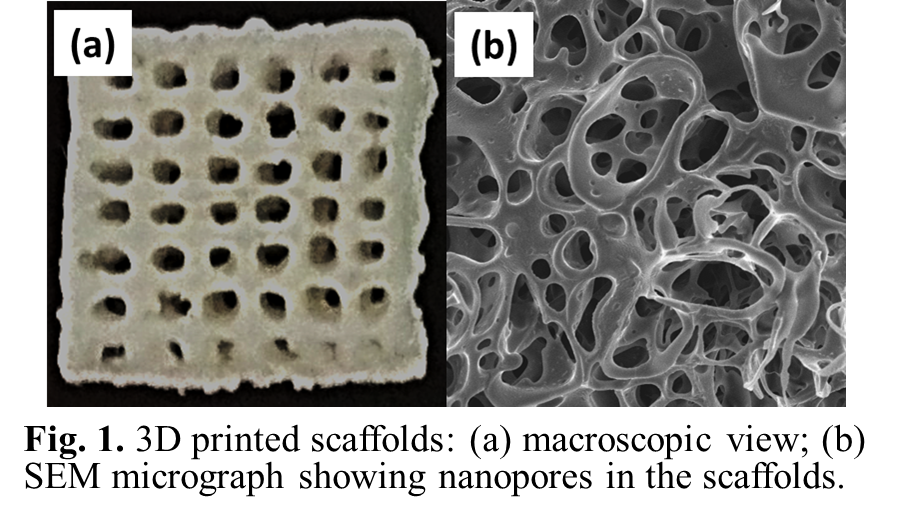
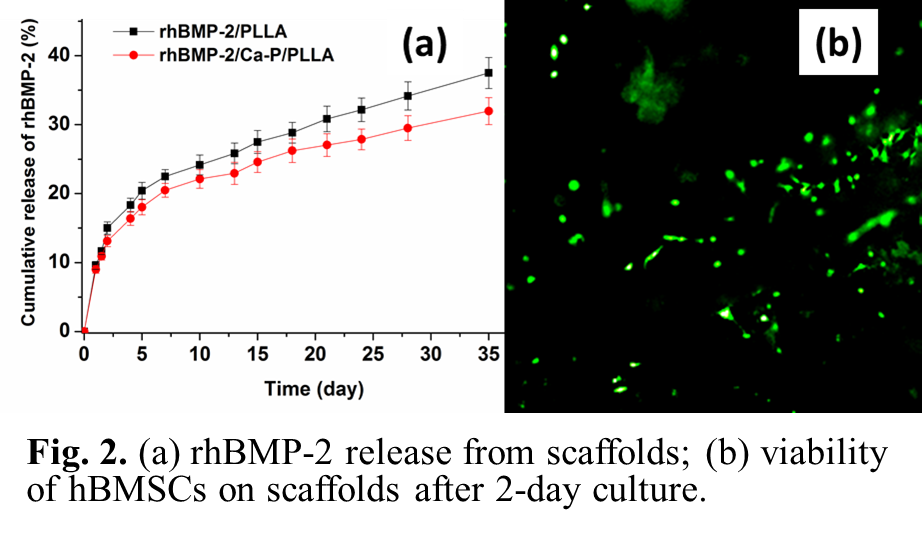
Results and Discussion: Three types of bone tissue engineering scaffolds were made: osteoinductive scaffolds (“P1”, rhBMP-2/PLLA), osteoconductive scaffolds (“P2”, Ca-P/PLLA), and bicomponent scaffolds (“P3”, rhBMP-2/PLLA/Ca-P/PLLA; consisting of two layers of P1 and two layers of P2, alternately). 3D scaffolds were built up layer-by-layer according to CAD files. Each scaffold had a four-layer structure (Fig.1a). Numerous nano-size pores were observed on strut surface (Fig.1b). Compression tests revealed P2 and P3 scaffolds had higher compressive strength than P1 scaffolds. rhBMP-2 released from P1 scaffolds had a controlled release profile, including an initial fast release, followed by a slower and sustained release (Fig.2a). In comparison, P3 scaffolds provided a slower release of rhBMP-2 (Fig.2a). In in vitro experiments, after 2-day hBMSC culture, nearly all stained hBMSCs on P1, P2 and P3 scaffolds were alive (green in color), indicating that the scaffolds were non-toxic (Fig.2b). The expression of F-actin and vinculin adhesion plaque in hBMSCs on P1 and P2 scaffolds was intensively up-regulated compared to scaffolds without rhBMP-2 or Ca-P incorporation, suggesting that the presence of rhBMP-2 and Ca-P from scaffolds facilitated cell adhesion. After 7-day culture, individual hBMSCs on scaffolds showed a normal phenotype and morphology with an expanded polygonal shape and numerous filapodia.
Conclusion: A 3D printing technique was established for scaffold fabrication by using an inexpensive commercial 3D printer. Both BMP-2 and Ca-P nanoparticles could be incorporated in 3D printed scaffolds. BMP-2 was released from scaffolds in a controlled manner and retained bioactivity. Released BMP-2 and Ca2+ ions promoted the proliferation and adhesion of hBMSCs.
References:
[1] S.W. Teoh, et al., Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling, J Biomed Mater Res, 2001, 55:203 - 216
[2] B. Duan, M. Wang, Customized Ca-P/PHBV nanocomposite scaffolds for bone tissue engineering: design, fabrication, surface modification and sustained release of growth factor, J R Soc. Interface, 2010, 7, S615.
[3] C. Wang, M. Wang, Dual-source dual-power electrospinning and characteristics of multifunctional scaffolds for bone tissue engineering, J Mater Sci Mater Med, 2012, 23:2381-97.
[4] C. Wang, M. Wang, Cost-effective 3D printing of tissue engineering scaffolds, Proceedings of ICBP2015, Singapore, 2015