Introduction: It is know that the physicochemical properties of the biomaterial surface influence the biological response of an implant, and as Ti is considered a bioinert metal, it is necessary to use surface modification methods in order to improve its osseointegration [1]-[3]. It is important to develop materials and surfaces with adequate corrosion resistance, which is a necessary condition for the biocompatibility [4],[5]. Considering that nanotubes layers on the Ti surface can enhance the osseointegration [6],[7], the aim of the present work was to obtain nanotubes on the Ti surface and to study its electrochemical behavior in a simulated physiological media.
Materials and Methods: Nanotubes were obtained on Ti (grade II) surface by anodic oxidation, using a two electrode system [8],[9]. The electrochemical anodization was performed applying 10V/1h, 20V/1h or 20V/2h, using as working electrolyte Glycerol / H2O / NaF [8].
The electrochemical analyses were carried out using a standard three-electrode cell, a saturated Ag/AgCl electrode as reference, and Ringer Physiological solution [8]-[10]. The open circuit potential values, Eoc, were monitored until 144h of immersion, and the polarization measurements were performed from -0.100 mV up to 1.5V (vs Eoc) at 1mV/s.
Results and Discussion:
After anodization, it was obtained well-defined nanotubes on the entire surface of all samples, with average diameter of 45nm for 10V and 90nm for 20V, and length of 1µm for 1h and 2 µm for 2h, consistent with previous work[8]. Exemplifing, in the Fig.1 are presented SEM micrographs for 10V and 20V for 1 h.

Figure 1 - SEM micrographs of nanotubes layer formed at (a) 10V / 1h and (b) 20V / 1h.
The Eoc profiles were similar (Fig.2a), indicating that the nanotubes films formed are not suffering spontaneous dissolution and also that the different diameters and lengths are not influencing their electrochemical properties. These values are higher than the values found by Oliveira & Guastaldi[5] for cp Ti polished after 360h immersion in Ringer's solution: -200mV (vs SCE).
The voltammetric profiles are similar for all surfaces (Fig. 2b), with low current values, on the order of µA, without the occurrence of transpassivation in the potential range studied, indicating the presence of a barrier-type oxide film[5], that is probably related to the oxide layer at the bottom of the nanotubes. The shift of the corrosion potential from -600 mV (as polished surface) to -300mV (nanotubes surfaces) indicates that the presence of the nanotubes is improving the Ti corrosion resistance. The variation in diameter and length of the nanotubes did not influence their electrochemical behavior, confirming the results obtained in the Eoc measurements.
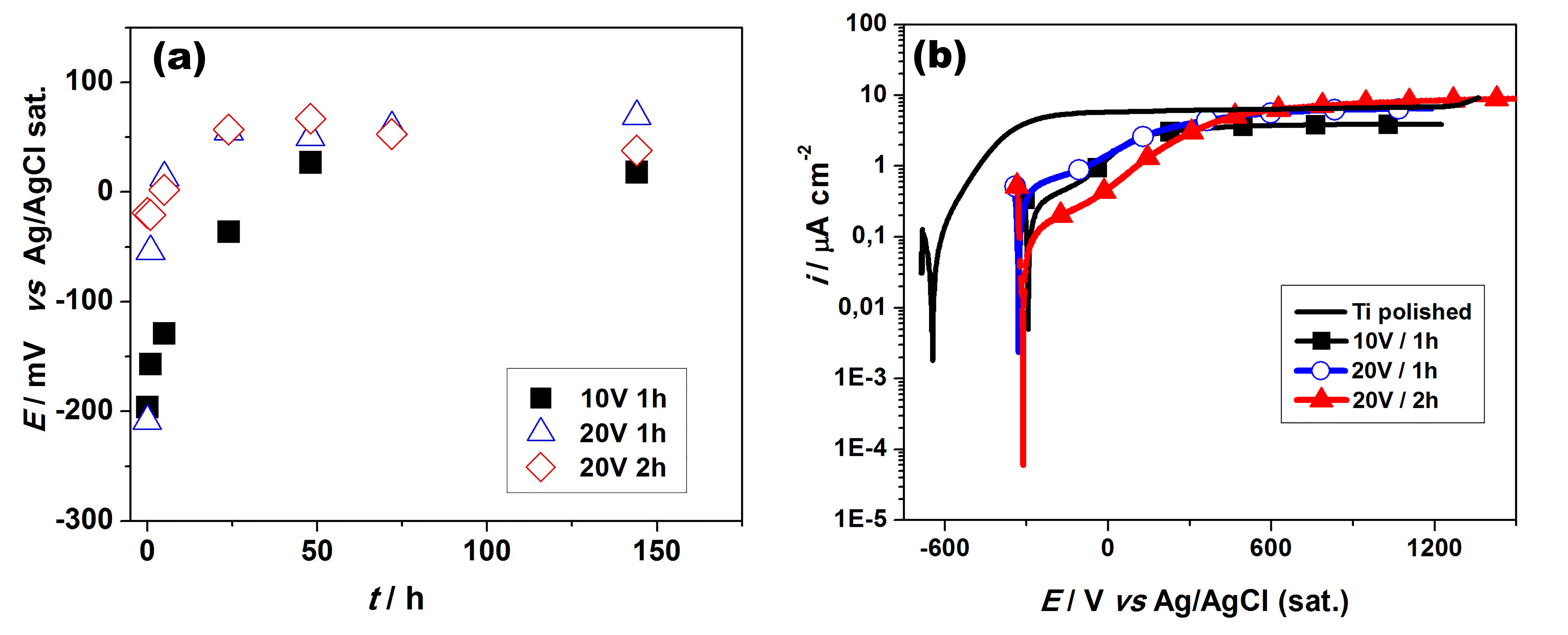
Figure 2 – (a) Open-circuit potential measurements and (b) potentiodynamic polarization for Ti with nanotubes layer formed at 10V / 1h, 20V / 1h, and 20V / 2h.
Conclusion: The nanostructured layers formed on pure Ti with different diameters and length of the nanotubes improved the electrochemical stability and corrosion resistance of the Ti.
The authors are grateful to FAPESP for scholarships (proc. 2012/11350-0) and grants (2012/01652-9).
References:
[1] BAUER S., SCHMUKI P., VON DER MARK K., PARK J., “Engineering biocompatible implant surfaces Part I: Materials and surfaces.” Progress in Materials Science, 58, 261–326, 2013.
[2] HUANG H., WUC., SUN Y., YANG W., LEE T. “Surface nanotopography of an anodized Ti–6Al–7Nb alloy enhances cell growth”Journal of Alloys and Compounds, 615, S648–S654, 2014.
[3] PARK H.H., PARK I.S., KIM K.S., JEON W.Y., PARK B.K., KIM H.S., BAE T.S., LEE M.H. “Bioactive and electrochemical characterization of TiO2 nanotubes on titanium via anodic oxidation”. Electrochimica Acta 55, 6109–6114, 2010.
[4] OLIVEIRA N.T.C., ALEIXO G., CARAM R., GUASTALDI A.C., “Development of Ti-Mo alloys for biomedical applications: microstructure and electrochemical characterization” J Journal of Materials Science and Engineering A, 452/3, 727-731, 2007.
[5] OLIVEIRA N.T.C.; GUASTALDI A.C.; “Electrochemical stability and corrosion resistance of Ti-Mo alloys for biomedical applications” Acta Biomaterialia, Vol. 5, 399, 405, 2009.
[6] NIINOMI M., KURODA D., FUKUNAGA K., MORINAGA M., KATO Y., YASHIRO T., SUZUKI A., “Corrosion wear fracture of new β type biomedical titanium alloys” Journal of Materials Science and Engineering A, 263, 193-199, 1999.
[7] OKAZAKI Y., RAO S., TATEISHI T., ITO Y., “Cytocompatibility of various metals and desenvelopment of new titanium alloys for medical implants” Journal of Materials Science and Engineering A, 243, 250-256, 1998.
[8] CÓRDOBA-TORRES P.; OLIVEIRA N.T.C.; BOLFARINI C.; ROCHE V.; NOGUEIRA R.P.; “Electrochemical impedance analysis of TiO2 nanotube porous layers based on an alternative representation of impedance data” Journal of Electroanalytical Chemistry, 737, 54-64, 2015.
[9] OLIVEIRA N.T.C; VERDERIO J.F.; BOLFARINI C.; “Obtaining self-organized nanotubes on biomedical Ti–Mo alloys” Electrochemistry Communications, 35, 139–141, 2013.
[10] OLIVEIRA N.T.C.; BIAGGIO S.R.; NASCENTE P.A.P., PIAZZA S., SUNSERI C. & Di QUARTO F. “The effect of thickness on the composition of passive films on a Ti-50Zr at% alloy”. Electrochim. Acta, 51¸ No. 17, 3506-3515, 2006.