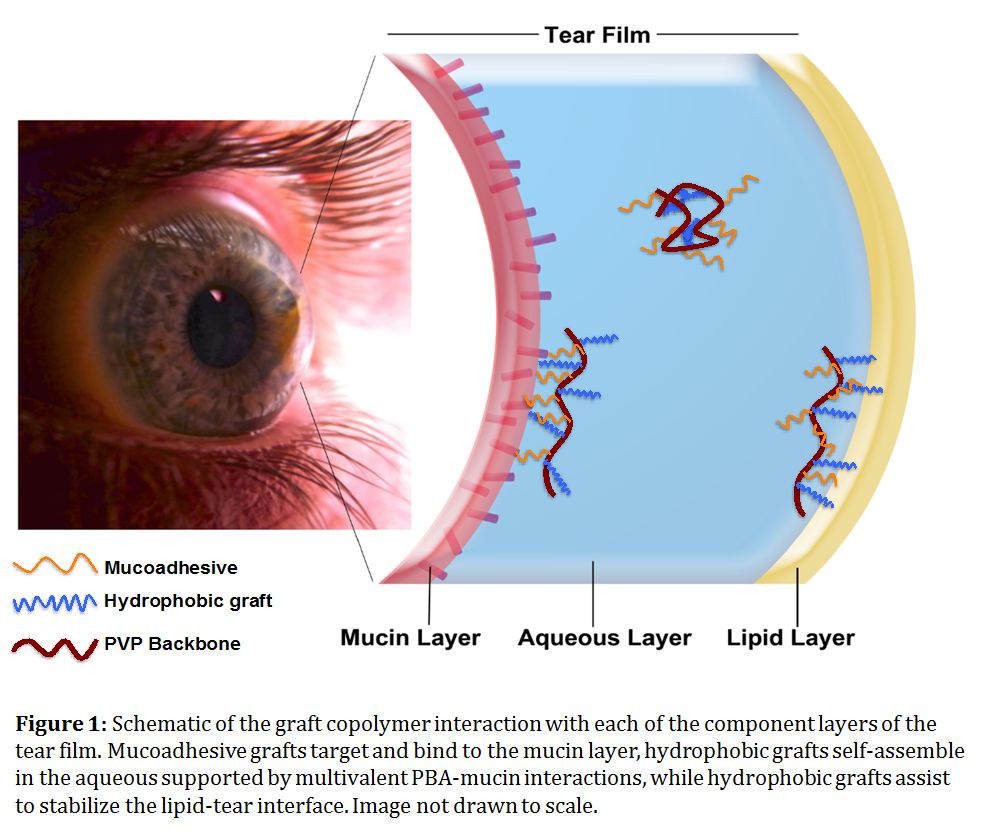
Introduction: Effective drug delivery to the eye is challenging due to the multiple biological barriers promoting rapid clearance, with as little as ~5% of drugs administered via eyedrops reaching the cornea[1]. Shear-associative hydrogels cross-linked via physical interactions that can be broken under stress offer a potential solution given that (a) they are easily to administer (b) they can self-lubricate blinking and (c) they can be engineered with different viscosities to control release kinetics[2],[3]. Adding a mucoadhesive functionality offers further potential to enhance the residence time in the tear film and thus reduce the required frequency of eyedrops. Herein, we describe a physically-gelling hydrogel formulation based on poly(vinylpyrrolidone) (PVP) chemically modified with both hydrophobes (C8 – C18) and phenylboronic acid (PBA) mucoadhesive groups[4] with potential to interact with each layer of the tear film (mucoadhesion to cornea, self-associations between hydrophobes and free mucin-PBA interactions in the aqueous, and hydrophobe assembly in the lipid layer, Fig. 1).
Materials and Methods: Functional PVP was prepared via copolymerization of N-vinylpyrrolidone (VP) with N-vinylformamide (VF) followed by hydrolysis of VF residues to primary amines. Alkylchloride (C8 – C18) hydrophobes were then grafted to 15-20 mol% of the free amine groups via condensation, after which 4-formylphenylboronic acid was grafted to the remaining amine groups via reductive amination using sodium cyanoborohydride. Shear thinning and mucoadhesion were measured using rheology, while transmittance was measured using UV/vis spectrophotometry. Drug release kinetics of doxorubicin hydrochloride were measured using UV/vis spectrophotometry. In vitro cytotoxicity to human corneal epithelial cells (HCECs) was assessed using an MTT assay. Anterior segment biocompatibility was tested by administering 0.1 mL of 15 wt% polymer solution to the eyes of New Zealand White rabbits and scoring ocular responses using Draize scoring.
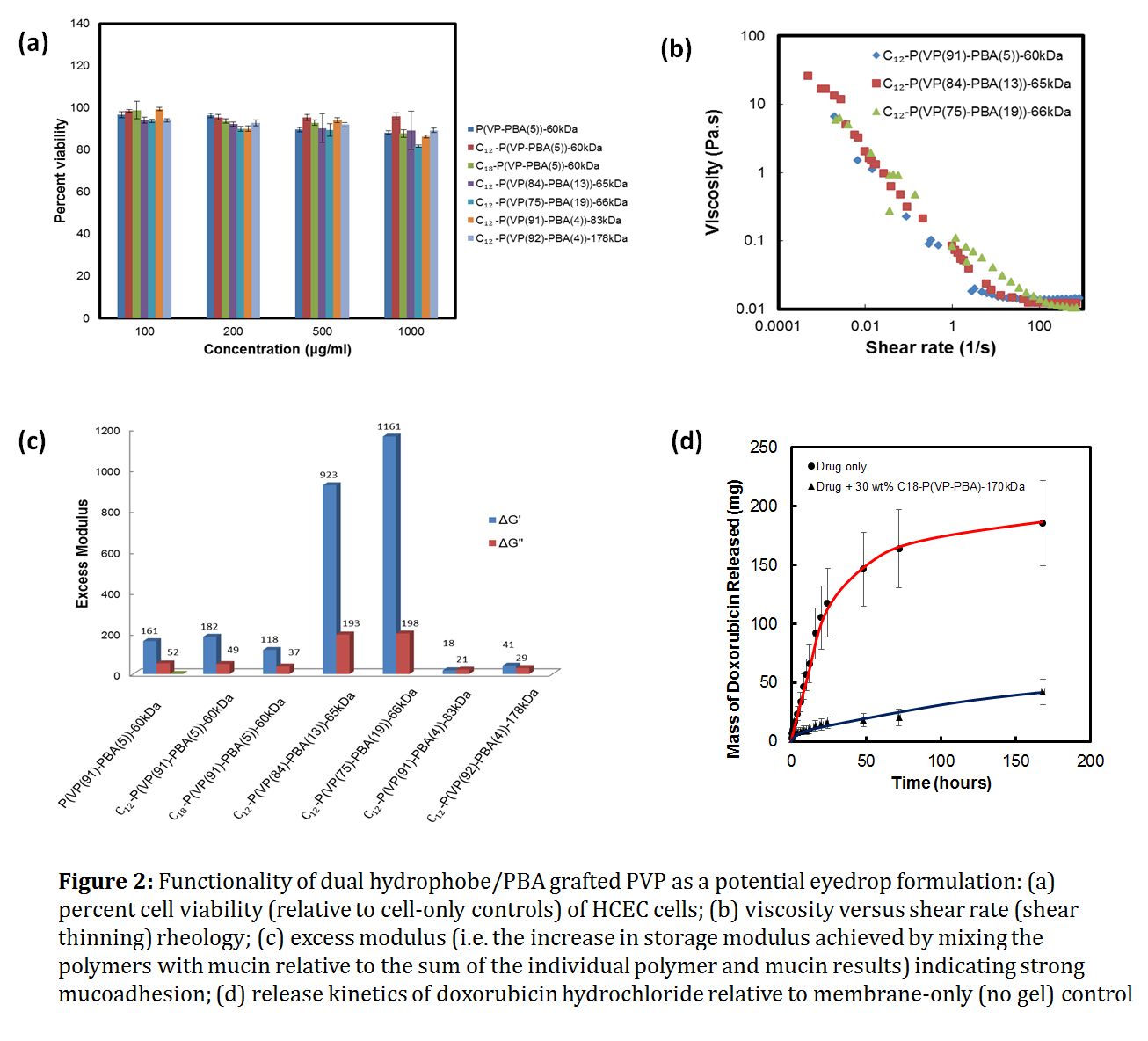
Results and Discussion: Polymers containing up to 2.5 mol% hydrophobes and 19 mol% PBA showed transmissions (>90%), refractive indices (~1.348), and cytocompatibilities (>90% cell viability to HCECs, Fig. 2a) suitable for ophthalmic use, even at high concentrations (15 wt%). The polymers are highly shear-thinning, with 3-4 decades of viscosity between zero and infinite shear relatively independent of the PBA content (Fig. 2b). The excess storage modulus associated with polymer-mucin interactions is as high as >1 kPa, suggesting strong mucoadhesion (Fig. 2c). Formulations effectively prolonged release of doxorubicin hydrochloride over ~1 week (Fig. 2d).
Draize scoring indicated no significant conjunctival edema or redness, secretion, corneal opacity, or iris involvement; indeed, the treated eyes showed less inflammation than the control eyes, suggesting potential benefits for dry eye treatment.
Conclusion: Hydrophobically and PBA-modified PVP can form a highly shear-sensitive self-associative gel that remains transparent, demonstrates both shear thinning and mucoadhesive potential, facilitates controlled drug release, and is biologically well-tolerated in vitro and in vivo. We anticipate this polymer may offer particular utility in eyedrops for either prolonged drug release to the anterior segment and/or alone as a dry eye therapeutic.
20/20: NSERC Ophthalmic Materials Research Network (funding)
References:
[1] Kompella, U.B.; Kadam, R.S.; Lee, V.H.L. Therapeutic Deliv. 2010, 1, 435
[2] Annaka, M.; Mortensen, K.; Vigild, M.E.; Matsuura, T.; Tsuji, S.; Ueda, T.; Tsujinaka, H. Biomacromolecules 2011, 12, 4011
[3] Sheikholeslami, P.; Muirhead, B.; Baek, D.S.H.; Wang, H.; Zhao, X.; Sivakumaran, D.; Boyd, S.; Sheardown, H.; Hoare, T. Exper. Eye Res. 2015, 137, 18
[4] Winblade, N.D.; Nikolic, I.D.; Hoffman, A.S.; Hubbell, J.A. Biomacromolecules 2000, 1, 523