Introduction: Endotracheal tubes (ETTs) provide a route for nosocomial organisms to colonize the lower respiratory tract and cause ventilator-associated pneumonia (VAP), which is the most common nosocomial infection in the intensive care unit. The antimicrobial ETTs are developed by the combination of the release of an antimicrobial polypeptide (AMP) with non-fouling surface modification technology.
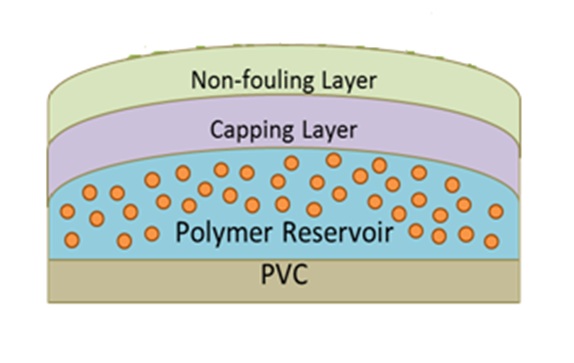
Materials and Methods: The unmodified ETTs were made of PVC by Teleflex. The AMP (KSL-W) was developed and provided by the USAISR-DTRD[1]. The formulated ETT had three layers: the methacrylate copolymer reservoir layer containing the KSL-W AMP, then a methacrylate copolymer capping layer, and finally a non-fouling, sulfobetaine methacrylate (SBMA) copolymer as the top coat layer (Figure 1). Automatic dip coating machine was used to make these coatings on full ETT devices.
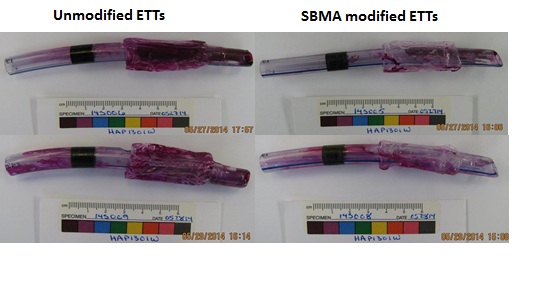
Results and Discussion: Zwitterionic SBMA modified ETTs: The non-fouling SBMA modified ETT was compared with ETTs modified by hydrophilic polymers including non-ionic, cationic, and anionic hydrophilic polymers, hydrophobic polymers, and superhydrophobic coatings made of boehmite[2] or commercial NeverWet (from Rust-OleumÒ). From the I-125 labelled fibrinogen and mucin analysis, SBMA modified ETT showed super fibrinogen resistance and better to similar mucin resistance to other modified surfaces. In vivo test was 5hrs of intubation in sheep. After extubated and stained with periodic acid, the non-fouling SBMA modified ETT showed significant reduction in the mucus/sputum adsorption to 58% and 81% compared to 20% and 35% for the control (Figure 2).
Antimicrobial ETTs: When non-fouling SBMA was combined with KSL-W AMP to make three layers coating on ETTs, they demonstrated moderate resistance to mucin attachment in the presence of the AMP (36-55%).Antimicrobial testing against S. aureus and P. aeruginosa, showed substantially reduce the attachment of the test organisms by 2-4 log10 compared to unmodified ETTs for a period of 72 hours (Figure 3). The AMP release showed the AMP was rapid released within the first 72h and followed by a slower release up to 5d.

A sheep model was developed based on the previously published model by Berra et al[3]. to study the antimicrobial activity of this AMP-releasing ETT. The result demonstrated that there were substantially fewer culturable microbial cells (2 to 4 log10) for all of the trachea, bronchial, and lung cultures of the antimicrobial ETTs.
Conclusion: The antimicrobial ETT with AMP-releasing developed by Teleflex has the ability to substantially reduce microbial colonization of the lower trachea, bronchi, and lungs in sheep.
Studies was funded by a grant from USAMRAA (award no. W81XWH-12-2-0084)
References:
[1] Concannon et al. J. Med Micro. 2003, 52,1083.
[2] Kim et al. Nano Letters 2013, 13, 1793.
[3] Berra et al. Anesthesiology. 2004, 100, 1446.