Introduction: PI3K / mTOR signaling is dysregulated in over 60% of clinical breast cancers across all three major clinical subtypes (ER+, HER2+, and triple negative breast cancers), driving tumor cell growth, survival, metabolism, and invasion[1],[2]. The distal effector of this pathway, mTOR, is found within two functionally distinct complexes, mTORC1 and mTORC2. Our group’s recent work has implicated a distinct physiologic role for mTORC2 in PI3K hyperactivated breast cancers, where it drives tumor survival and motility[3],[4]. While small molecules exist which can inhibit mTORC1 (e.g. rapalogues) or both mTORC1 and mTORC2 (e.g. mTOR kinase inhibitors), no current technology exists which can preferentially inhibit mTORC2 activity. Thus, siRNA nanoparticles (si-NPs) were optimized through a combinatorial approach to enable potent RNA interference (RNAi) of mTORC2 signaling through target gene silencing of the mTORC2-specific cofactor RICTOR.
Materials and Methods: Thirty si-NP formulations were synthesized with different ratios and compositions of core-forming and PEGylated, corona-forming polymers. These siRNA-loaded polyplexes were assessed for size, zeta potential, stability, pH-dependent membrane disruption as a gauge of endosomal escape, flow cytometry for cell uptake, and gene silencing and cytotoxicity in vitro. Pharmacokinetics, biodistribution, and target gene silencing were tested in vivo in mice harboring MDA-MB-231 xenografts in each mammary fat pad. Anti-tumor efficacy of Rictor silencing was tested in vivo on MDA-MB-231 (TNBC) or MDA-MB-361 (HER2+ BC) mammary xenografts.
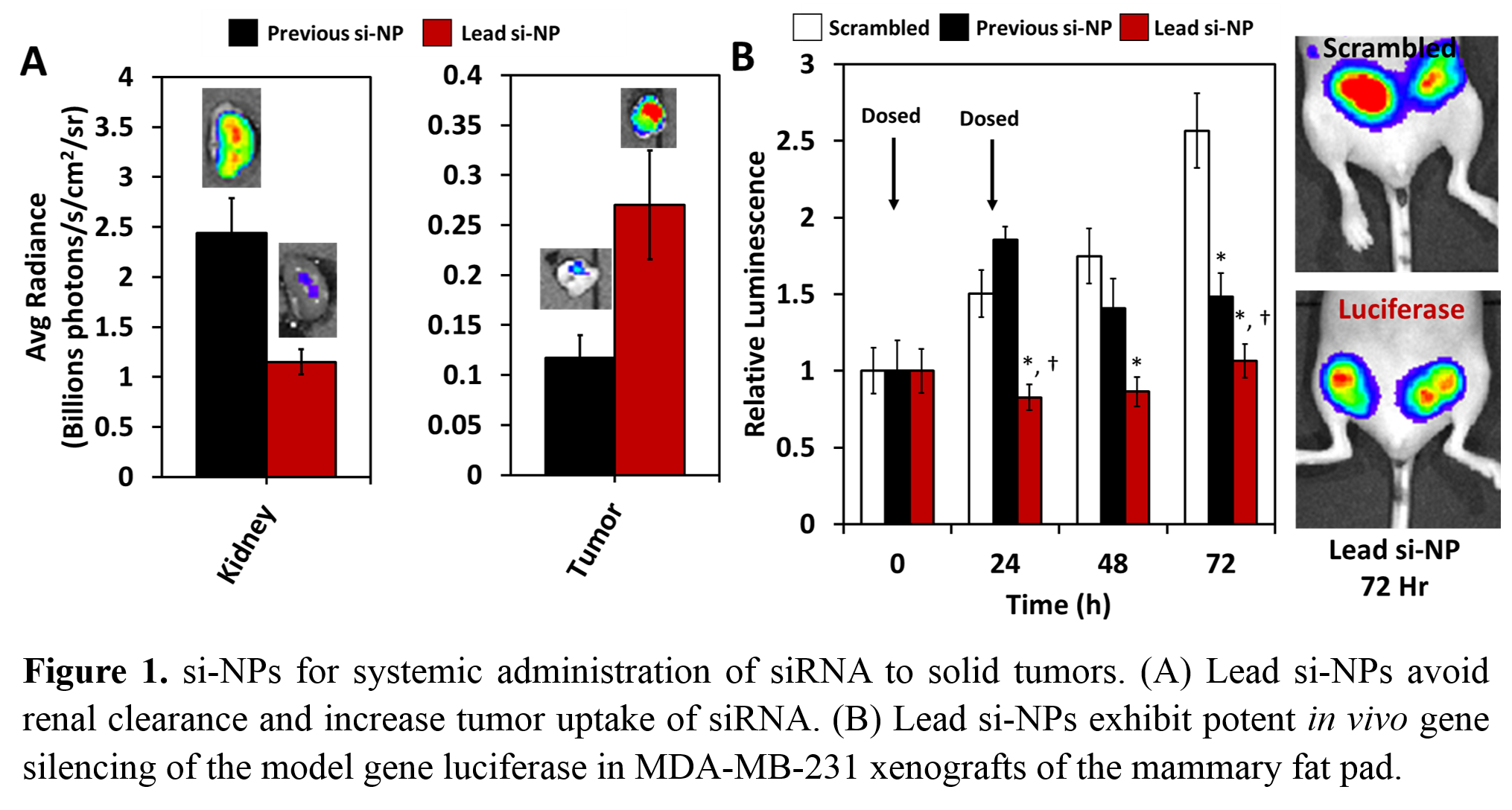
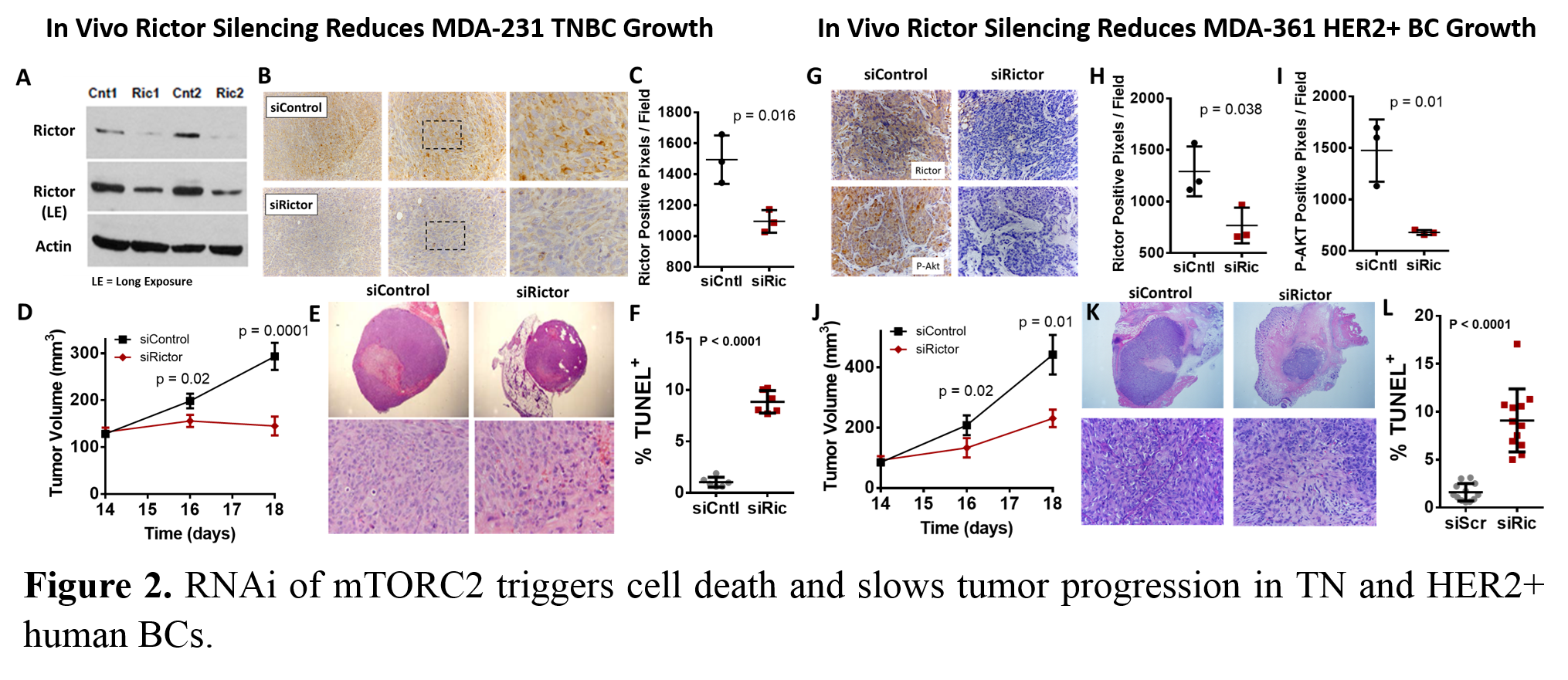
Results and Discussion: Screening of 30 si-NP formulations yielded a lead candidate that was small (~100 nm), stable in size and cargo unpackaging, efficiently internalized by BC cells, potent in pH-dependent membrane disruption screens, and effective at gene silencing in vitro (~90% silencing of model gene luciferase at a dose that did not cause significant toxicity). Lead si-NPs exhibited greater in vivo circulation (2-fold higher AUC), reduced renal clearance (~0.5-fold), and increased tumor uptake (2.6-fold) compared to our previous lead formulation[5] (Figure 1a). The desirable pharmacokinetics of the new si-NPs led to ~60% model gene (luciferase) silencing in tumors in vivo (Figure 1b). RNAi of mTORC2 through RICTOR gene silencing significantly decreased tumor growth in both TNBCs (MDA-MB-231) and HER2+ BCs (MDA-MB-361) in vivo (Figure 2a-l). Potent RICTOR gene silencing in tumors was confirmed by western blot and IHC for RICTOR and P-Akt (mTORC2 substrate) (Figure 2b, g). The reduction in tumor growth was mechanistically tied to 5-10 fold increases in apoptosis in RICTOR siRNA treated animals relative to controls (Figure 2f, l).
Conclusion: A new si-NP formulation was developed with superior in vivo pharmacokinetics and tumor bioactivity. This formulation was successfully applied for RNAi of Rictor. The approach enabled selective inhibition of mTORC2 which is not possible with small molecule drugs. Efficacy studies confirmed the therapeutic value of selective inhibition of mTORC2 which may be associated with less toxicity relative to pan-mTORC inhibition, as mTORC1 is known to have roles in metabolism and other healthy cell maintenance. Ongoing studies will further elucidate the distinct roles of mTORC1 and mTORC2 in tumor growth and study drug combinations with mTORC2 RNAi.
NIH (R21EB012750 and R01EB019409); NSF GRFP (TW, MJ, and TK)
References:
[1] Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4452-4461
[2] Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: Role in tumor progression and therapeutic implications in breast cancer. Breast cancer research : BCR. 2011;13:224
[3] Morrison MM, Young CD, Wang S, Sobolik T, Sanchez VM, Hicks DJ, Cook RS, Brantley-Sieders DM. Mtor directs breast morphogenesis through the pkc-alpha-rac1 signaling axis. PLoS Genet. 2015;11:e1005291
[4] Morrison MM, Jones BA, Hicks DJ, Sanchez VM, Estrada MV, Young CD, Williams MM, Sarbassov DD, Muller WJ, Brantley-Sieders DM, Cook RS. Rictor/mTORC2 drives progression and therapeutic resistance of HER2-amplified breast cancers. In Review at Journal of Clinical Investigation.
[5] Nelson CE, Kintzing JR, Hanna A, Shannon JM, Gupta MK, Duvall CL. Balancing cationic and hydrophobic content of pegylated sirna polyplexes enhances endosome escape, stability, blood circulation time, and bioactivity in vivo. ACS Nano. 2013;7:8870-8880