Introduction: Aqueous two phase system consisting of poly(ethylene glycol) (PEG) and polysaccharides have been investigated for easy purification of proteins and cell particles[1]. In our previous studies, PEG and hyaluronic acid (HA)–based two phase systems were examined to control insulin partition, and the system made us to achieve the controlled release of insulin[2]. In addition, PEG-grafted HA
(PEG-g-HA, Fig.1) was found to form a heterogeneous domain structure, in which insulin was partitioned and continuously released[3]. However, the effectiveness of in vivo controlled release and physiological activity of insulin in relation to the chemical composition of PEG-g-HA are still unclear.
In this study, we examined preparative condition of PEG-g-HA to easily modulate the graft number of PEG. Correlation between insulin release profile and in vivo activity in terms of blood glucose level was investigated by subcutaneous injection of PEG-g-HA containing insulin to rats.
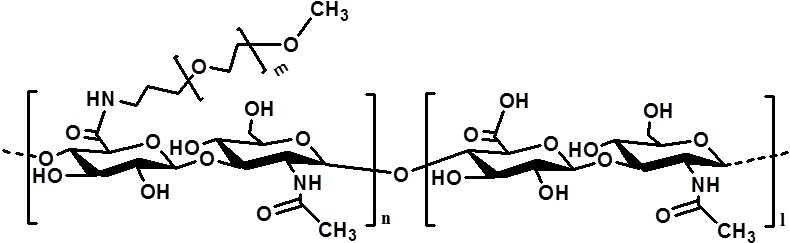
Materials and Methods: PEG-g-HA was prepared by a condensation reaction between HA and a mono-amino PEG .
Transmittance of the PEG-g-HA dissolved in a phosphate-buffered saline was measured. In vitro insulin release tests were performed by using a fluorescently labeled insulin that was mixed with the PEG-g-HA solution, and the release behavior was monitored by a fluorescence measurement.
Subcutaneous administration of insulin/PEG-g-HA solution to rat was performed, and serum glucose was determined.
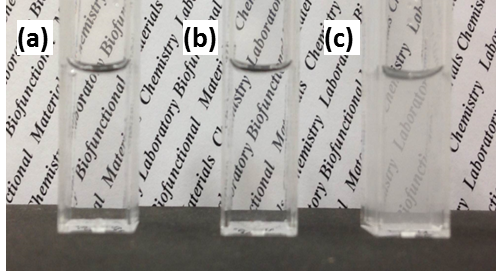
Results and Discussion: The number of PEG was controlled by just feed ratio of PEG and HA. PEG-g-HA solution became turbid, although HA and PEG solutions were transparent (Fig. 2), indicating the heterogeneous structure of PEG segment was formed in PEG-g-HA solution.
In vitro insulin release behavior showed in a sustained manner. This result suggests that the exercise of PEG microdomain contributed to the partition of insulin to the microdomain. In addition, from the results of subcutaneous administration to rats, blood glucose level decreased, and the low level was kept longer than the control samples (insulin, insulin/PEG). These results strongly suggest that the sustained release of insulin from the PEG microdomain was also observed in blood, and the insulin activity was maintained due to the partition of insulin to the PEG-surrounded environment.
Conclusion: PEG-g-HA formed heterogenous structure of PEG microdomain that selectively locates insulin even in the blood. The PEG-g-HA solution maintained the insulin activity with controlled release function.
Acknowledgemet: We thank to Prof. Hidetoshi ARIMA of Kumamoto University for In vivo insulin release examination.
Prof. Hidetoshi ARIMA of Kumamoto University
References:
[1] Albertsson, P. A., Adv. Protein Chem. 1970, 24, 309–341.
[2] Moriyama, K.; Yui, N., J. Controlled Release 1996, 42, 237-248.
[3] Moriyama, K.; Ooya, T.; Yui, N., J. Controlled Release 1999, 59, 77-86.