Fibronectin regulates enhanced wear protection of lubricin and mimetic lubricin during shear
-
1
University of Illinois at Urbana-Champaign, Department of Chemical and Biomolecular Engineering, United States
-
2
Cornell University, Department of Materials Science and Engineering, United States
-
3
Cornell University, Department of Biomedical Engineering, United States
Introduction: Synthetic biolubricants have been widely investigated as coatings for implants and the treatment of diseases, such as osteoarthritis. However, particularly for osteoarthritis, there have been few effective lubricants. A mucin known as lubricin, found in the synovial fluid, is one compound responsible for the low friction and is known to provide wear protection of cartilage[1],[2]. However, lubricin is expensive to extract or synthesize, hence a suitable lubricin-mimetic molecule is still to be created.
In this study, we use Atomic force microscopy (AFM) and the Surface Forces Apparatus (SFA) to characterize the molecular characteristics, and the normal and friction forces of a lubricin-mimetic pAA-graft-PEG copolymer (pAA-62kDa, PEG-2kDa abbreviated pAA-g-PEG) interacting with fibronectin (Fn), a structural protein found in the superficial zone of cartilage[3],[4]. The normal and the lateral (friction) forces of pAA-g-PEG polymers were recorded using the SFA, in the presence and absence of Fn coating.
Collectively, these results have allowed for molecular and microscopic characterization of the pAA-g-PEG interactions with surfaces, gaining insight inot both the lubrication mechanisms and the interactions of the biomimetic polymer with tissue through Fn, indicating that our proposed lubricin-mimetic lubricant might be a promising affordable alternative to lubricin.
Materials and Methods: Poly(acrylic acid) was synthesized by RAFT polymerization using acrylic acid (AA). The pAA-graft-PEG (pAA-g-PEG) copolymer was synthesized by polymer analogous conjugation of monoamine-functionalized PEG to the pAA backbone.
AFM measurements were performed in air using a commercial AFM. Pyramidal SiO2 probes were used for intermittent contact mode imaging.
Normal and friction forces between two mimLUB-coated mica surfaces were measured using the SFA using well-established procedures[5].
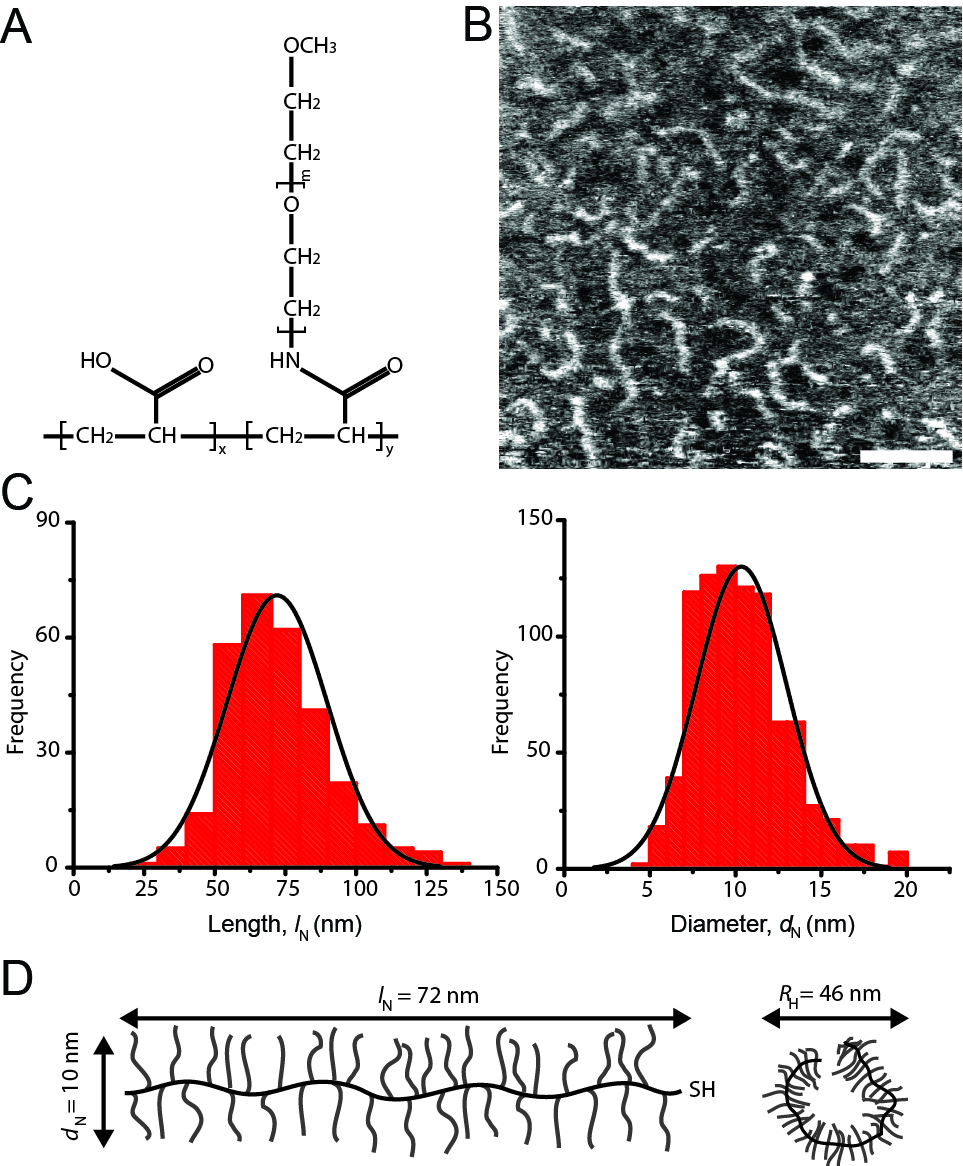
Results and Discussion: Our AFM data indicated that the pAA-g-PEG molecules had an average contour length and a diameter of 72nm and 10nm, respectively, as indicated in Figure 1.
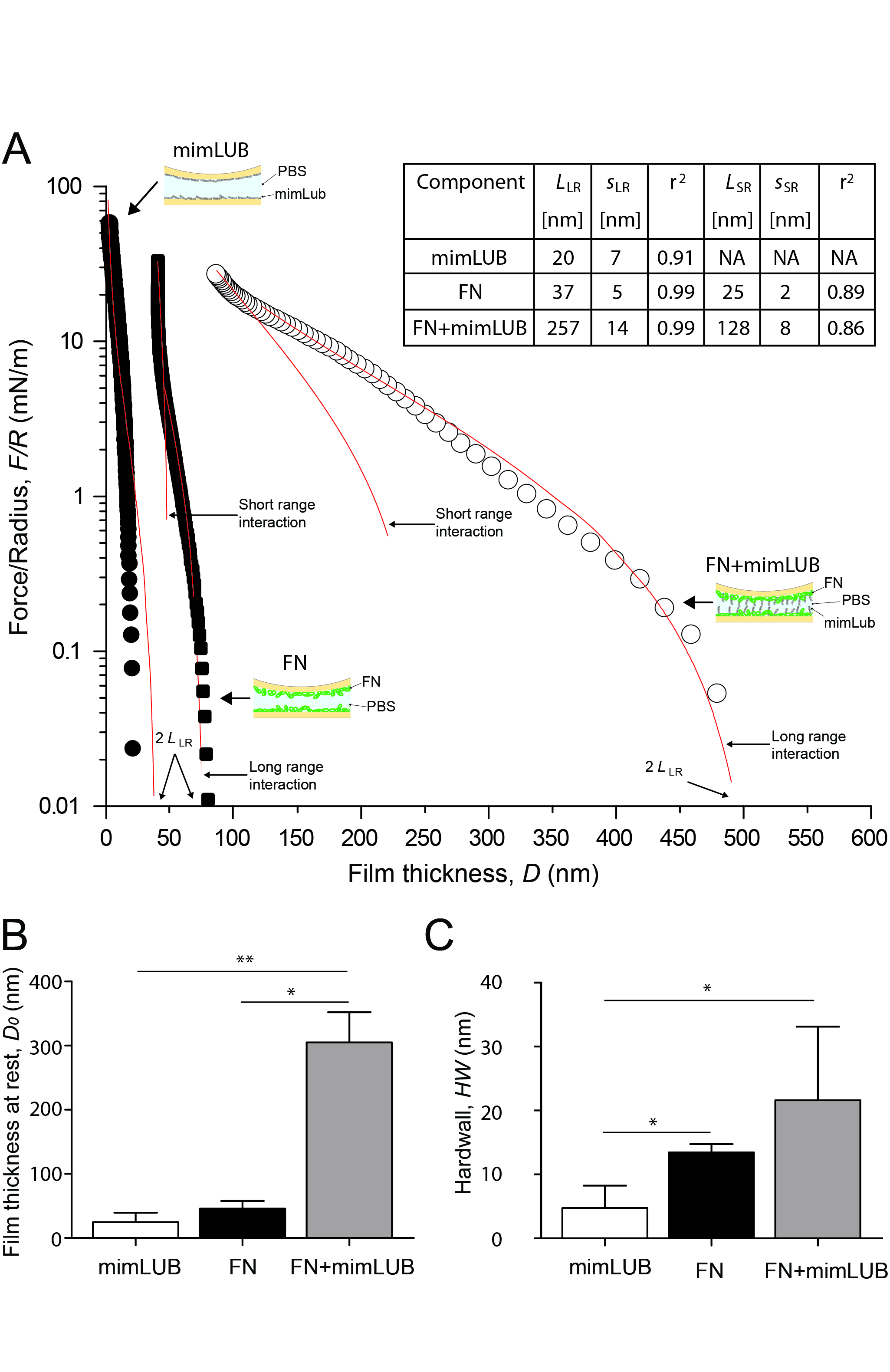
Our SFA data showed that the pAA-PEG polymer was only weakly adsorbed onto (negatively charged) bare mica surfaces but became firmly attached when Fn was added as a polymer linker, as presented in Figure 2.
All our friction data exhibited (i) low friction coefficients (µ ≈ 0.25) up to applied pressures of 3 MPa and (ii) Amonton's like behavior, as shown in Figure 3.
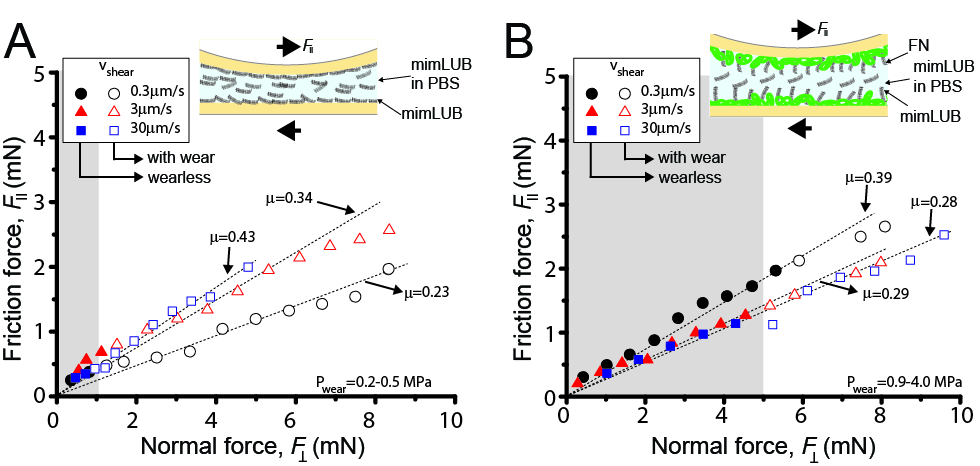
Conclusion: We report the design and characterization of a biomimetic lubricant (mimLUB) whose molecular architecture was inspired by lubricin, and the role of fibronectin in mediating mimLUB lubrication of ideal mica substrates. Although poor lubrication was achieved with mimLUB alone, enhanced wearless friction and surface protection were measured up to contact pressures of 4MPa when mimLUB was combined with FN. This effect was attributed to FN-mediated stronger binding of mimLUB to mica, which facilitates the formation (and retention) of a dense, robust, and highly hydrated (through PEG) repulsive brush that prevents polymer chain interpenetration when surfaces are sheared past each other, even under high pressures. As FN is abundant in the superficial zone of articular cartilage, our findings suggest that mimLUB could be a potential effective alternative to natural lubricin for the treatment of damaged cartilage surfaces or artificial joints, such as in the knee or the hip.
NSF under award DMR-1352299; CONACYT under award 308671; NIH under award 1R01AR066667-01; NIH Award Number 1S10RR023748-01
References:
[1] Swann, D. a; Sotman, S.; Dixon, M.; Brooks, C. The Isolation and Partial Characterization of the Major Glycoprotein (LGP-I) from the Articular Lubricating Fraction from Bovine Synovial Fluid. Biochem. J. 1977, 161, 473–485.
[2] Swann, D. a; Slayter, H. S.; Silver, F. H. The Molecular Structure of Lubricating Glycoprotein-I, the Boundary Lubricant for Articular Cartilage. J. Biol. Chem. 1981, 256, 5921–5925.
[3] Balazs, E. a. The Role of Hyaluronan in the Structure and Function of the Biomatrix of Connective Tissues. Struct. Chem. 2009, 20, 233–243.
[4] Chevalier, X. Fibronectin, Cartilage, and Osteoarthritis. Semin. Arthritis Rheum. 1993, 22, 307–318.
[5] Gourdon, D.; Yasa, M.; Alig, A. R. G.; Li, Y.; Safinya, C. R.; Israelachvili, J. N. Mechanical and Structural Properties of BaCrO 4 Nanorod Films under Confinement and Shear. Adv. Funct. Mater. 2004, 14, 239–242
Keywords:
Extracellular Matrix,
Biomimetic,
Surface modification,
wear
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
New Frontier Oral
Topic:
Surface and interfacial characterization
Citation:
Andresen Eguiluz
R,
Brown
CN,
Tan
M,
Cook
SG,
Pacifici
NJ,
Putnam
DA,
Bonassar
LJ and
Gourdon
D
(2016). Fibronectin regulates enhanced wear protection of lubricin and mimetic lubricin during shear.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02127
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Roberto Andresen Eguiluz, University of Illinois at Urbana-Champaign, Department of Chemical and Biomolecular Engineering, Urbana, IL, United States, Email1
Dr. Cory N Brown, Cornell University, Department of Materials Science and Engineering, Ithaca, NY, United States, Email2
Dr. Mingchee Tan, Cornell University, Department of Biomedical Engineering, Ithaca, NY, United States, Email3
Dr. Sierra G Cook, Cornell University, Department of Materials Science and Engineering, Ithaca, NY, United States, Email4
Dr. David A Putnam, Cornell University, Department of Biomedical Engineering, Ithaca, NY, United States, Email5
Dr. Lawrence J Bonassar, Cornell University, Department of Biomedical Engineering, Ithaca, NY, United States, Email6
Dr. Delphine Gourdon, Cornell University, Department of Materials Science and Engineering, Ithaca, NY, United States, Email7