Introduction: Gold nanoparticles (AuNPs) have found numerous applications in nanomedicine, in view of their robustness, ease of functionalization and low toxicity[1],[2]. Our group has demonstrated that near-infrared (NIR) femtosecond (fs) laser excitation of 100 nm citrate-capped AuNPs enables high cell membrane perforation, while maintaining high cell viability and enabling transfection of DNA plasmids into cancer cells[3]. Such laser irradiation of AuNPs minimizes the heat absorption from AuNPs and biological tissues since they absorb energy very weakly in the NIR range[4],[5]. The amplification of the electromagnetic field around the irradiated AuNP can cause the generation of nanoplasma leading to a nanobubble, thus inducing cell perforation without AuNP fragmentation[3],[6]-[8].
Since selective targeting of diseased cells can increase therapeutic efficacy and limit off-target adverse effects, the next step for selective cell optoporation is to develop stable AuNPs which labeled targeted cells without affecting surrounding non-targeted cells. Here the receptor CD44 strongly expressed by cancer cells was used as a model for selective targeting and optoporation.
Materials: Monoclonal anti-CD44 antibodies (Abs) from abcam. Orthopyridyl-disulfide-poly(ethylene glycol) (5kDa)-N-hydroxysuccinimide (OPSS-PEG-NHS) and HS-PEG (5kDa) from Nanocs. Citrate-capped AuNPs (50 μg/mL, 100 nm in diameter) from Nanopartz. HS-PEG (2kDa), PBS, Lucifer Yellow (LY) and DAPI from Sigma-Aldrich. Tetramethylrhodamine-wheat germ agglutinin (WGA) and Alexa 488-Abs from Life Technologies.
Methods: Abs were conjugated to OPSS-PEG-NHS (OPSS-PEG-Ab). AuNPs were functionalized with 0.01 OPSS-PEG-Ab/nm2 and 5 µM HS-PEG[9]. The stability of functionalized AuNPs (fAuNPs) was evaluated by UV-visible spectroscopy and zeta potential measurements. Cells were incubated for 3 h with 8 μg/mL fAuNPs, washed with PBS, incubated with LY and treated with a 45 fs laser at 800 nm (Spitfire, Spectra Physics). Two hours after the laser treatment, the cells were washed with PBS, fixed and stained with DAPI.
Results and Discussion: The fAuNPs with HS-PEG (5kDa) were colloidally stable in cell culture medium containing serum proteins, while fAuNPs with HS-PEG (2kDa) were unstable.
Selective targeting with stable fAuNPs was confirmed by immunofluorescence, darkfield and fluorescence imaging, flow cytometry and SEM on targeted CD44+ human cells (MDA-MB-231 breast cancer and ARPE-19 retinal pigmented epithelium) and on non-targeted CD44- mouse 661W photoreceptors (Fig. 1). The fAuNPs attached mostly as single particles 115 times more to targeted cells than to non-targeted cells.
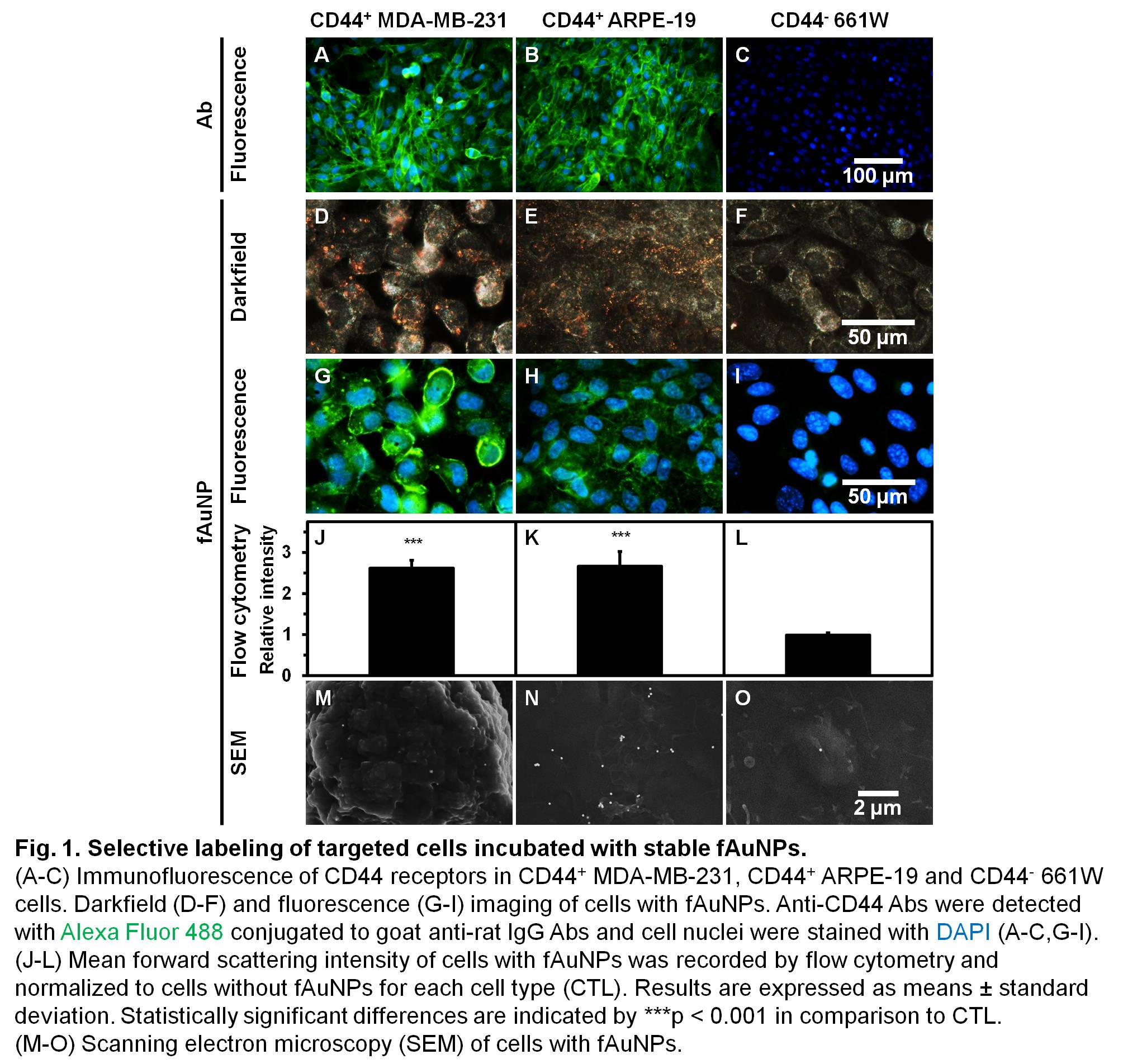
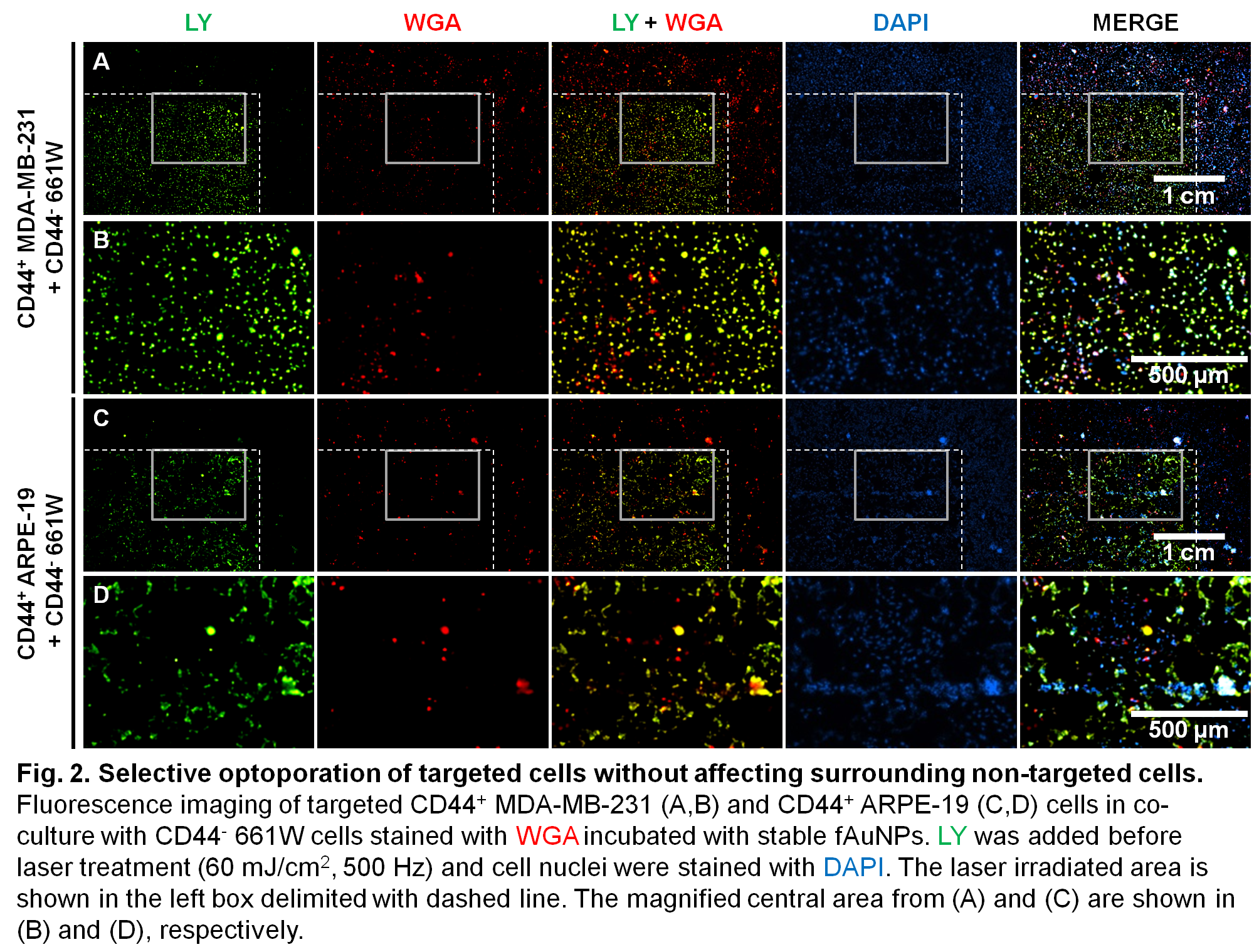
Selective optoporation of targeted cells was demonstrated with stable fAuNPs enhancing fs laser without affecting surrounding non-targeted cells (Fig. 2 and 3). Perforated cells in the laser-irradiated area were mainly targeted cells which accumulated LY, while untreated cells remained non-perforated[10].
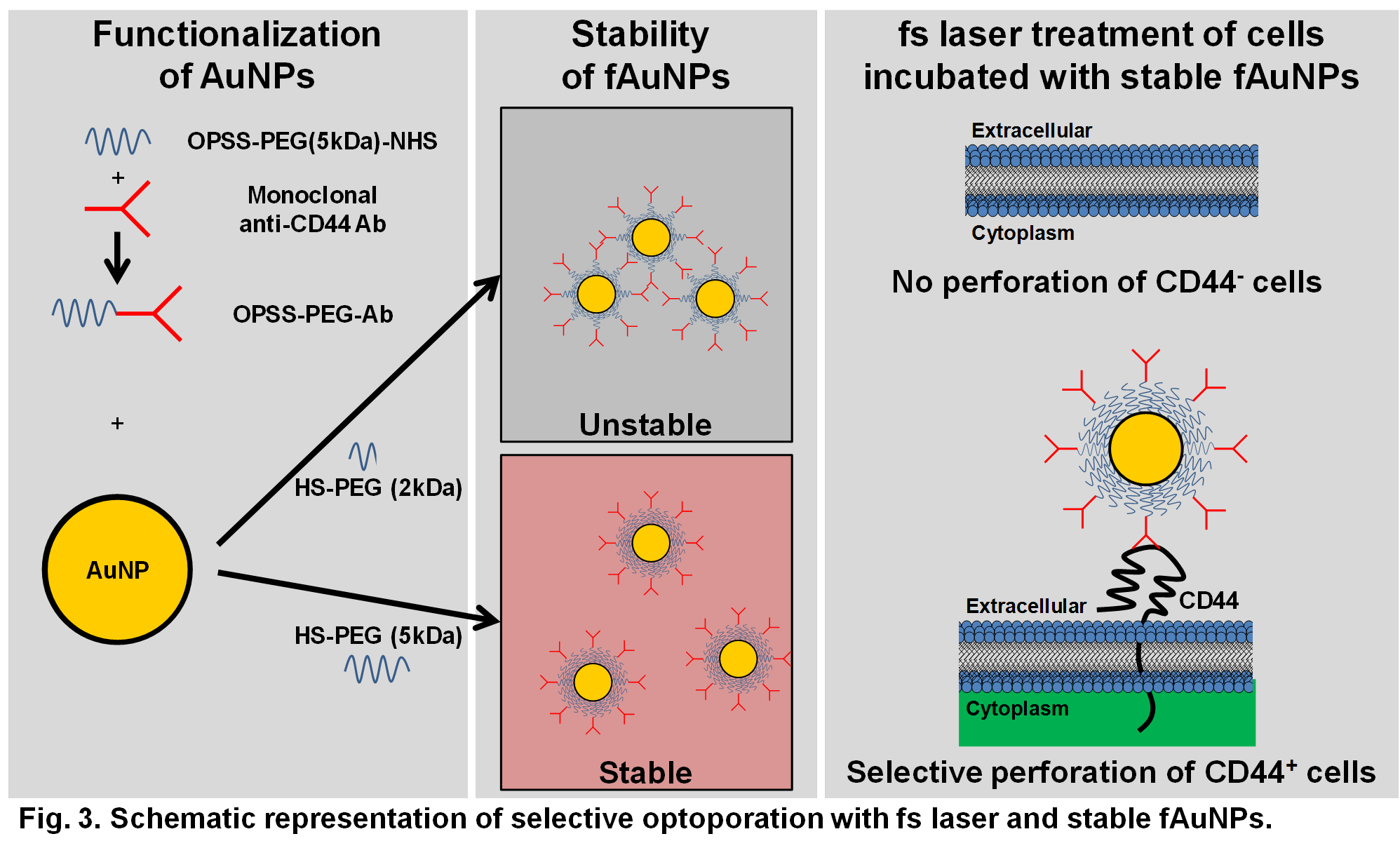
Conclusion: The proposed novel highly versatile treatment paradigm can be adapted to target and perforate other cell populations by adapting to desired biomarkers. Since living biological tissues absorb energy very weakly in the NIR range, the developed non-invasive tool may provide a safe, cost-effective clinically relevant approach to ablate pathologically deregulated cells and limit complications associated with surgical interventions.
This work was supported by Le Fonds de recherche du Québec, Consortium québécois sur la découverte du médicament (CQDM), the Natural Science and Engineering Research Council of Canada (NSERC) and Canadian Institutes of Health Research (CIHR). EB received funding from Fonds de recherche du Québec – Santé and CB acknowledges funding from the EU under a Marie Curie Fellowship, FP7-PEOPLE-2013-IOF, project reference 624888. David Rioux, Sergiy Patskovsky, Rémi Lachaine, Alexandra Thibeault-Eybalin, Ariel Wilson, Flavie Lavoie-Cardinal and Yves Drolet are acknowledged for technical assistance and fruitful discussions. The authors also thank Danièle Gagné and Gaël Dulude from the IRIC flow cytometry platform for assistance with flow cytometry.
References:
[1] X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed (2007) Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy, Nanomedicine 2(5):681−693.
[2] K. Weintraub (2013) The new gold standard, Nature 495(7440):S14−S16.
[3] J. Baumgart, L. Humbert, É. Boulais, R. Lachaine, J.-J. Lebrun and M. Meunier (2012) Off-resonance plasmonic enhanced femtosecond laser optoporation and transfection of cancer cells, Biomaterials 33(7):2345−2350.
[4] É. Boulais, R. Lachaine and M. Meunier (2012) Plasma mediated off-resonance plasmonic enhanced ultrafast laser-induced nanocavitation, Nano Letters 12(9):4763−4769.
[5] R. Weissleder (2001) A clearer vision for in vivo imaging, Nature Biotechnology 19(4):316–317.
[6] E. Boulais, R. Lachaine, A. Hatef and M. Meunier (2013) Plasmonics for pulsed-laser cell nanosurgery: Fundamentals and applications, Journal of Photochemistry and Photobiology C: Photochemistry Reviews 17:26–49.
[7] C. Boutopoulos, E. Bergeron and M. Meunier (2015) Cell perforation mediated by plasmonic bubbles generated by a single near infrared femtosecond laser pulse, Journal of Biophotonics, in press. doi: 10.1002/jbio.201500135.
[8] W. Ding, E. Bergeron, R. Lachaine and M. Meunier, (2015) Nanomaterial-assisted light-induced poration and transfection of mammalian cells (chapter 16) IN: Applications of Nanoscience in Photomedicine, Editors: M. R. Hamblin and P. Avci, Sawston (Cambridge, United Kingdom): Woodhead Publishing Limited, pp. 331–376. doi: 10.1533/9781908818782.331, ISBN: 1907568670.
[9] S. Patskovsky, E. Bergeron, D. Rioux and M. Meunier (2015) Wide-field hyperspectral 3D imaging of functionalized gold nanoparticles targeting cancer cells by reflected light microscopy, Journal of Biophotonics 8(5):401–407.
[10] E. Bergeron, C. Boutopoulos, R. Martel, A. Torres, C. Rodriguez, J. Niskanen, J.-J. Lebrun, F. M. Winnik, P. Sapieha and M. Meunier (2015) Cell-specific optoporation with near-infrared ultrafast laser and functionalized gold nanoparticles, Nanoscale (in revision).