Introduction: Age-related macular degeneration (AMD) is the leading cause of blindness in Australia today[1]. The only way to save vision requires monthly injection to the back of the eye with biomacromolecular anti-angiogenics, such as bevacizumab (Avastin®) and ranibizumab (Lucentis®)[2]. This invasive procedure, with the potential for sight threatening complications, has obvious negative impacts on patient’s quality of life as well as posing a major burden on the global health care system. A drug delivery system that serves as a reservoir to enable sustained release or ‘on-demand’ release of therapeutic protein/antibody would be beneficial, since it will significantly reduce the number of injections into the back of the eye[3]. The release of therapeutic payloads from the drug reservoir can be modulated on demand by light, particularly due to its external spatiotemporal control[4].
Materials/Method: In order to fabricate a photo-responsive drug delivery system a thermo-rensponsive polymer was formulated into a hydrogel containing light sensitive nanoparticles (LSNPs) and therapeutic payloads (Fig. 1).
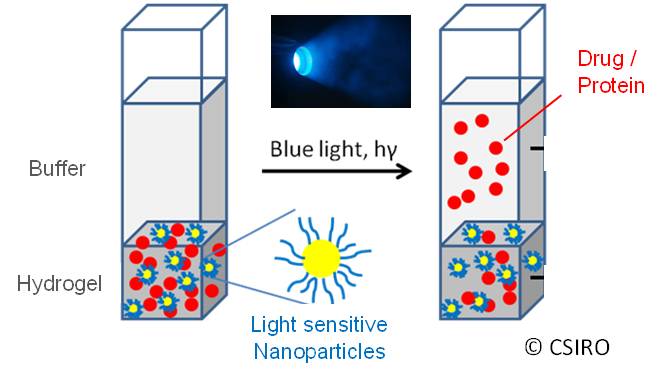
The release of different payloads, ranging from small molecules (doxorubicin, trimacinolone acetonide) to biomacromolecules (lysozyme, bovine serum albumin, and IgG/antibody), can be switched on and off upon exposure to visible light (Fig. 2).
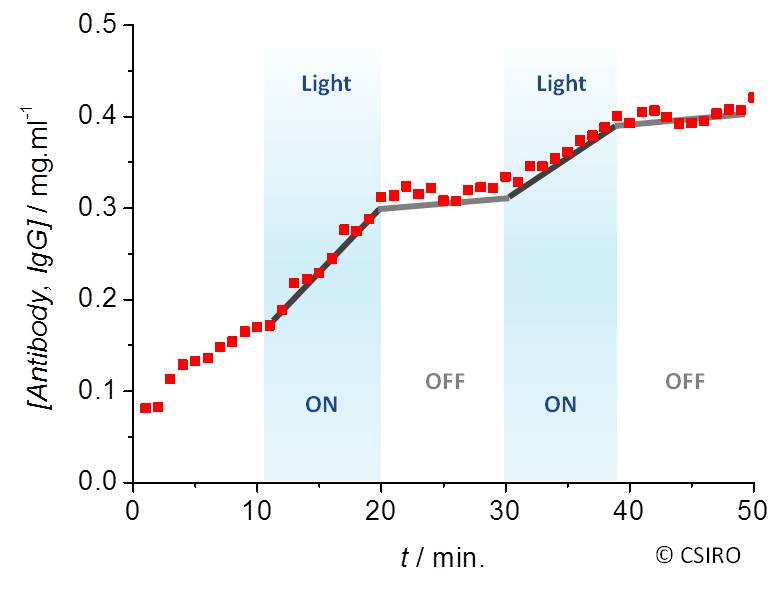
This hydrogel can be modified to a dispersion of microparticles or a thermo-reversible polymer solution for ocular injection, while additional surface coating can be applied for long-term sustained release.
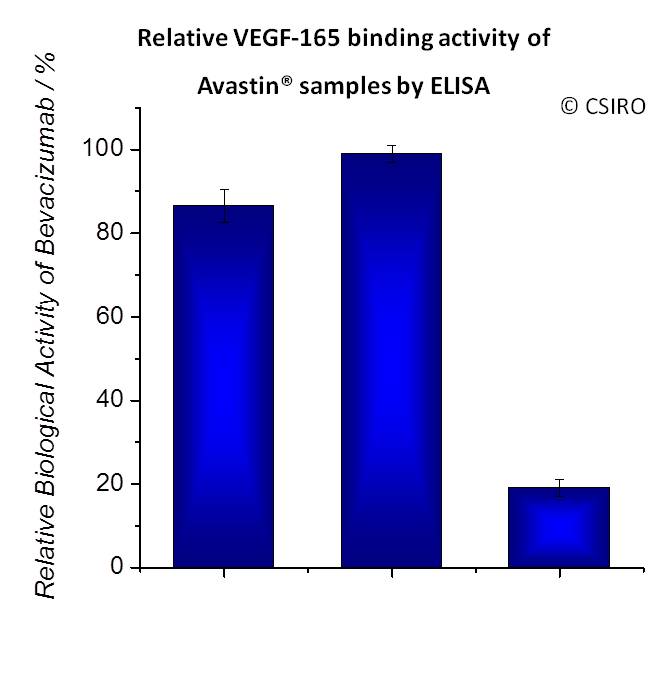
Results and Discussion: Released lysozyme, bevacizumab, and ranibizumab from this formulation exhibited above 85% biological activities after their exposure to light, in particular bevacizumab due to its binding affinity to human VEGF in the anti-angiogenic AMD therapy (Fig. 3).
The microparticulate formulation did not show in vitro toxicity to ocular cells, and was injected subconjunctivally through a 30-gauge needle to the rabbit. In this preliminary animal study the corneal and retinal examinations of the injected eye confirmed the biocompatibility of the formulation.
Conclusion: Due to its minimum toxicity and high versatility, this implantable photo-modulated drug delivery system has a potential to improve the treatment of ocular diseases. The release rate of the loaded drug can be tuned on demand by internal (e.g. LSNPs and polymer concentration) and external parameters (e.g. light intensity) for controlled dose.
Fengxiang Qie; Randy Suryadinata; Linda Ge; Tianwei Tan; Xiaojuan Hao; Mark Greaves
References:
[1] Hugh R Taylor, Jill E Keeffe, Hien T V Vu, Jie Jin Wang, Elena Rochtchina, M Lynne Pezzullo and Paul Mitchell, "Vision loss in Australia", Medical Journal of Australia, Vol. 182, June 2005
[2] Jayakrishna Ambati, John P. Atkinson and Bradley D. Gelfand, "Immunology of age-related macular degeneration", Nature Reviews Immunology, Vol. 13, June 2013
[3] Owen A. Anderson, James W.B. Bainbridge and David T. Shima, "Delivery of anti-angiogenic molecular therapies for retinal disease", Drug Discovery Today, Vol. 15, April 2010
[4] Salvatore Sortino, "Photoactivated nanomaterials for biomedical release applications", Journal of Materials Chemistry, Vol. 22, Nov. 2012