Modulating mucin hydration and lubrication by deglycosylation and polyethylene glycol binding
-
1
Massachusetts Institute of Technology, Biological Engineering, United States
-
2
Technische Universität München, Institute of Medical Engineering IMETUM and Department of Mechanical Engineering, Germany
-
3
Innovation Platforms Johnson & Johnson Family of Consumer Companies, Oral & Topical Healthcare R&D, United States
Introduction: A key property of mucin glycoproteins is their exceptional capacity to hydrate and lubricate surfaces. In vivo, mucins assemble into mucus hydrogels that cover the epithelium and protect it from dehydration and shear stress. A better understanding of the origin of these properties could lead to new treatment strategies for patients with poor mucus coverage, defective mucus production or glycosylation as caused by Sjögren syndrome, dry eye, or in the case of certain bacterial infections.
Materials and Methods: We use mucin coatings to show that mucin-associated glycans are essential for the formation of hydrated and lubricating layers. We compare native with deglycosylated mucins by analyzing their hydration and lubrication in the boundary and mixed lubrication regime. Hydration of the coating was deduced by comparing adsorbed mass as measured by quartz crystal microbalance with dissipation monitoring in the hydrated states, and elliposmetry in the dry state. Lubrication was measured using a commercial shear rheometer equipped with a tribology unit and the measurements were performed in a ball-on-cylinder geometry as described in reference [1]. Polyethylene glycol (PEG) was grafted to partially deglycosylated mucins using a sugar binding lectin to which PEG was conjugated.
Results and Discussion: The removal of glycans from the mucin resulted in a 3.5 fold decrease in hydration and an increase in friction by two orders of magnitude. These results suggest that the lubricative potential in the boundary and mixed lubrication regime of mucins is linked to the hydration. We countered this loss of function by grafting polyethylene glycol (PEG) molecules to defective mucins through lectin-glycan interactions. This lectin-PEG conjugation restored hydration and improved lubrication of the partially deglycosylated mucin coatings. Interrestingly, there was no effect of PEG alone or PEG conjugated to a lectin that did not bind to mucin glycans, meaning bindig of the conjugate to the surface was necessary for the effect to occur.
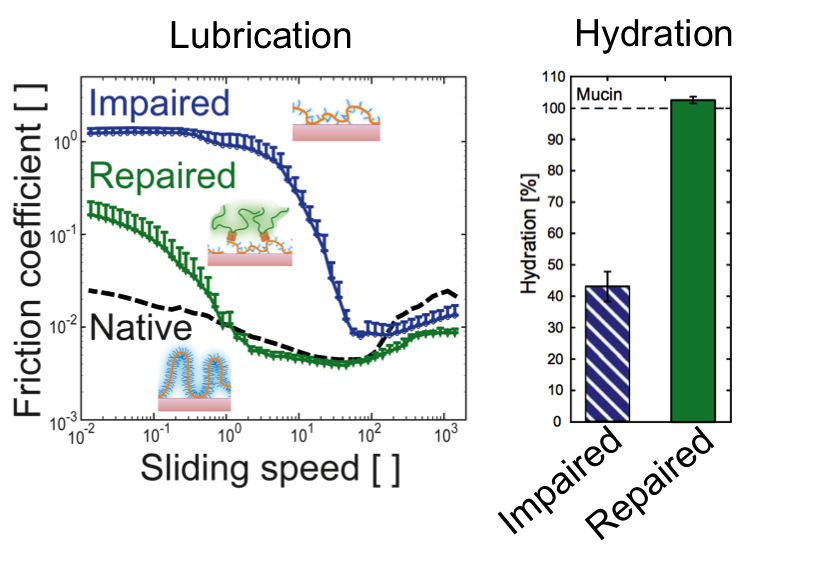
Conclusion: We have shown the importance of mucin glycans in mucin hydration and lubrication. It implies that alterations to the glycans in vivo through dysfunctional post-translational processing or enzymatic digestion of mucin glycans can result in deficient mucus layers [2]. We also show that topical treatment of the mucus with polymers could be a viable alternative to drugs for the local complementation of defective mucus layers.
References:
[1] K. Boettcher, S. Grumbein, U. Winkler, J. Nachtsheim, O. Lieleg, Rev. Sci. Instrum. 2014, 85, 093903.
[2] Thomas Crouzier, Kathrin Boettcher, Anthony R. Geonnott, Nicole L. Kavanaugh, Julie B. Hirsch, Katharina Ribbeck, and Oliver Lieleg, Modulating mucin hydration and lubrication by deglycosylation and polyethylene glycol binding, Accepted in Advanced Materials Interfaces
Keywords:
surface property,
biomedical application,
biomacromolecule,
bioinerface
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Interfacial phenomena
Citation:
Crouzier
T,
Boettcher
K,
Geonnotti
AR,
Kavanaugh
NL,
Hirsch
JB,
Ribbeck
K and
Lieleg
O
(2016). Modulating mucin hydration and lubrication by deglycosylation and polyethylene glycol binding.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.02047
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Kathrin Boettcher, Technische Universität München, Institute of Medical Engineering IMETUM and Department of Mechanical Engineering, Garching, Germany, kathrin.boettcher@tum.de
Dr. Anthony R Geonnotti, Innovation Platforms Johnson & Johnson Family of Consumer Companies, Oral & Topical Healthcare R&D, Skillman, NJ, United States, AGeonnot@its.jnj.com
Dr. Nicole L Kavanaugh, Massachusetts Institute of Technology, Biological Engineering, Cambirdge, MA, United States, nkav@mit.edu
Dr. Julie B Hirsch, Innovation Platforms Johnson & Johnson Family of Consumer Companies, Oral & Topical Healthcare R&D, Skillman, NJ, United States, JHirsch@its.jnj.com
Dr. Katharina Ribbeck, Massachusetts Institute of Technology, Biological Engineering, Cambirdge, MA, United States, ribbeck@mit.ed
Dr. Oliver Lieleg, Technische Universität München, Institute of Medical Engineering IMETUM and Department of Mechanical Engineering, Garching, Germany, oliver.lieleg@tum.de