Introduction: Encapsulation of drugs in nanoscale delivery vehicles can stabilize sensitive molecules, minimize systemic toxicity, and regulate the rate and location of drug release. Polymersomes are synthetic polymer vesicles with an aqueous core that can encapsulate large hydrophilic drugs, such as proteins. In this study, polymersomes with photoreversible cinnamoyl or coumarin crosslinks were prepared and their suitability for drug delivery was analyzed. Phototriggerable drug delivery would enable spatial and temporal control over the drug release based on clinical need and permit non-invasive control for ophthalmic applications, etc.
Materials and Methods: Cyclic carbonate monomers functionalized with cinnamoyl or coumarin were synthesized and copolymerized with trimethylene carbonate by melt polymerization at 115℃ using 5000 g/mol poly(ethylene glycol) as an initiator to prepare a series of diblock copolymers with 10000-20000 g/mol polycarbonate blocks and 15-25% functionalization. Polymersomes were prepared from the resulting polymers using either the traditional organic solvent injection method[1] or a protein-safe detergent removal method[2] and characterized using dynamic light scattering, UV-Vis spectroscopy, and confocal microscopy. The polymersomes’ membranes were stained with Nile Red and fluorescein isothiocyanate-tagged bovine serum albumin (FITC-BSA) was encapsulated as a model protein.
Results: Polymer vesicles ranging in size from 20 nm to 20 μm were prepared depending on the method used with a typical size of 200-500 nm. The photoreversibility of the crosslinking was determined to be ~75% by monitoring changes in UV-Vis absorbance. The impact of photocrosslinking on polymersome stability was assessed by diluting an aqueous suspension with THF (2 eq), which fully ruptured uncrosslinked polymersomes, but had no effect on polymersomes crosslinked with 365 nm UV (30 min at 200 mW/cm2). Following irradiation with 254 nm UV (30 min at 4 mW/cm2), the polymersomes once again ruptured in the presence of THF. In addition, polymersomes prepared at high ionic concentrations ruptured in hypotonic conditions following decrosslinking (Figure 1) due to osmotic pressure.
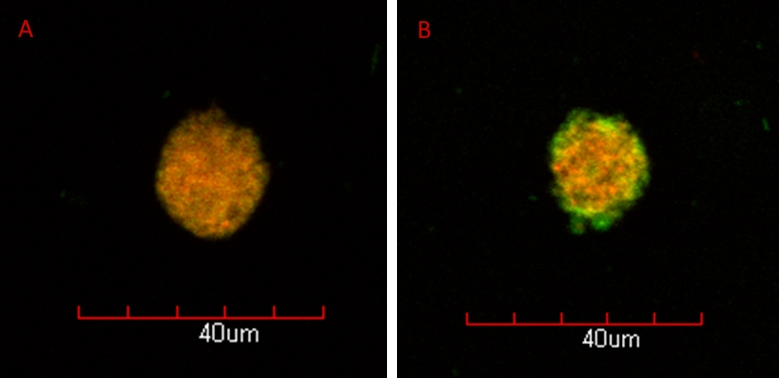
Figure 1: Confocal images of crosslinked (A) and decrosslinked (B) polymersomes (membranes stained red with Nile Red) in hypotonic conditions showing FITC-BSA (green) being released following decrosslinking
Discussion: The stability of the photocrosslinked polymersomes when exposed to THF and hypotonic conditions suggests that they would provide a highly robust encapsulation vehicle for proteins. Further, the rupturing of decrosslinked polymersomes when exposed to hypotonic conditions provides a mechanism for drug release following irradiation with either 254 nm UV or a 532 nm laser[3].
Conclusion: Photoreversible polymersomes were prepared and characterized. Phototriggerable control over polymersome stability and protein encapsulation was demonstrated and quantified.
Funding for this work was provided via the NSERC 2020 Ophthalmic Biomaterials Network
References:
[1] Meng, F., Hiemstra, C., Engbers, G. H. M. & Feijen, J. Biodegradable polymersomes. Macromolecules 36, 3004–3006 (2003).
[2] Marsden, H. R. et al. Detergent-aided polymersome preparation. Biomacromolecules 11, 833–838 (2010).
[3] Buckup, T., Southan, A., Kim, H.-C., Hampp, N. A. & Motzkus, M. Optimisation of two-photon induced cleavage of molecular linker systems for drug delivery. J. Photochem. Photobiol. A Chem. 210, 188–192 (2010).