Introduction: Biomimetics of the tumor-environment, an ecosystem, can be applied to create an ecological trap. An ecological trap is an environment of low quality for survival that is preferred by an organism over a better available environment. Carcinoma associated cancer cells (CAFs) produce an extracellular matrix (ECM) that exerts a high attraction to disseminated cancer cells, and is the perfect bait for an ecological trap. Microparticle encapsulated CAFs (MP[CAF]) can redirect adhesion of disseminated cancer cells from the peritoneal wall to the MP[CAF] surface to prevent peritoneal metastasis formation.
Materials and Methods: Encapsulation: CAFs cells are encapsulated into microparticles (MP[CAF] 500-700µm) by dripping a mixture of alginate (1.5%), gelatin (0.5%) and CAFs (2.106/ml) in to a CaCl2 bath (1.3%) though a needle (260µm inner diameter) surrounded by an airflow. MP[CAF] where layer-by-layer coated with poly-styrene sulfonate and poly-allyamine (PSS/PAH), with or without the incorporation of iron-oxide nanoparticles between the layers.
In vitro confrontation assay: MP[CAF] and MPs without CAFs where confronted with luciferase positive cancer cells (1.105) while shaking. After 48h adhesion the two types of MPs are magnetically separated and the adhesion of SK-OV-3 Luc on the MPs is measured through bioluminescence.
In vivo: MP[CAF] (200) are inserted through a small incision in to the abdominal cavity of nude mice. 1.105 ovarian cancer cells (SK-OV-3 Luc IP1) are injected. 24h later MPs with captured cancer cells are removed with a magnet. Sham threated mice underwent the same procedure but now MPs where inserted.
Results and Discussion: MP[CAF] are stable and encapsulated CAFs are viable and metabolic active for over 4 weeks. CAF-derived ECM proteins are retained by the PSS/PAH coating. In vitro confrontation show that cancer cells have a higher affinity for MP[CAF] compared to empty MP (fig 1A-B). Small animal MRI imaging shows distribution of MP throughout the abdominal cavity without attachment to intestinal organs and without signs of inflammatory reaction. MP[CAF] trap cancer cells and redirect adhesion away from the wound site. Removal of the MP[CAF] results in a delay peritoneal metastasis formation and a prolonged survival in mice (fig 2A-D).
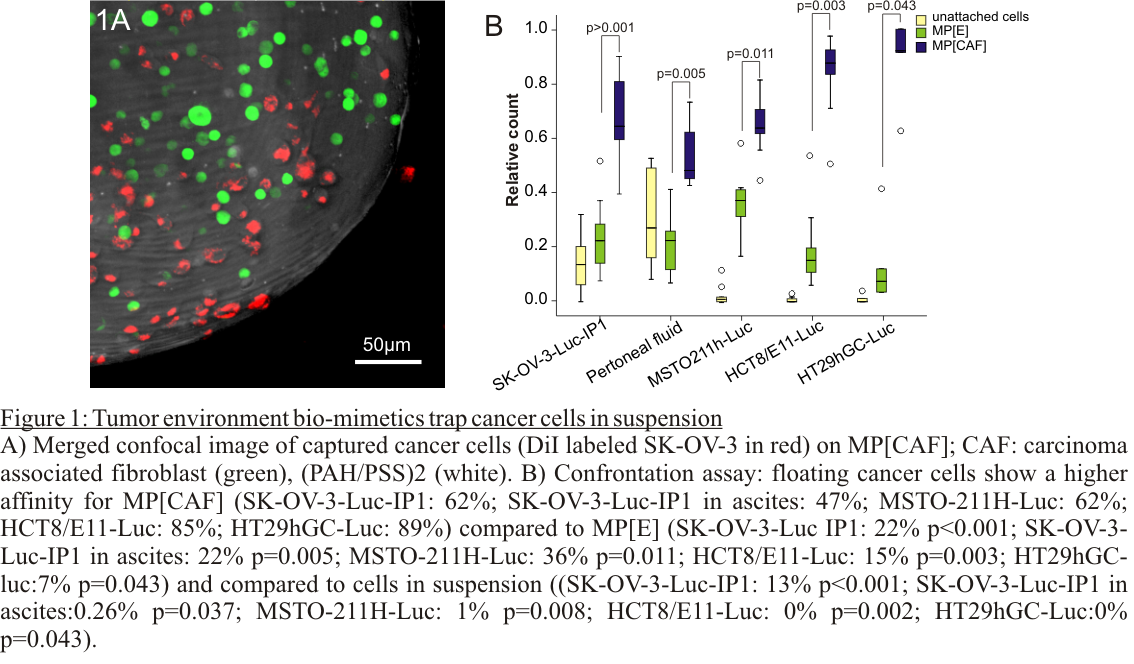
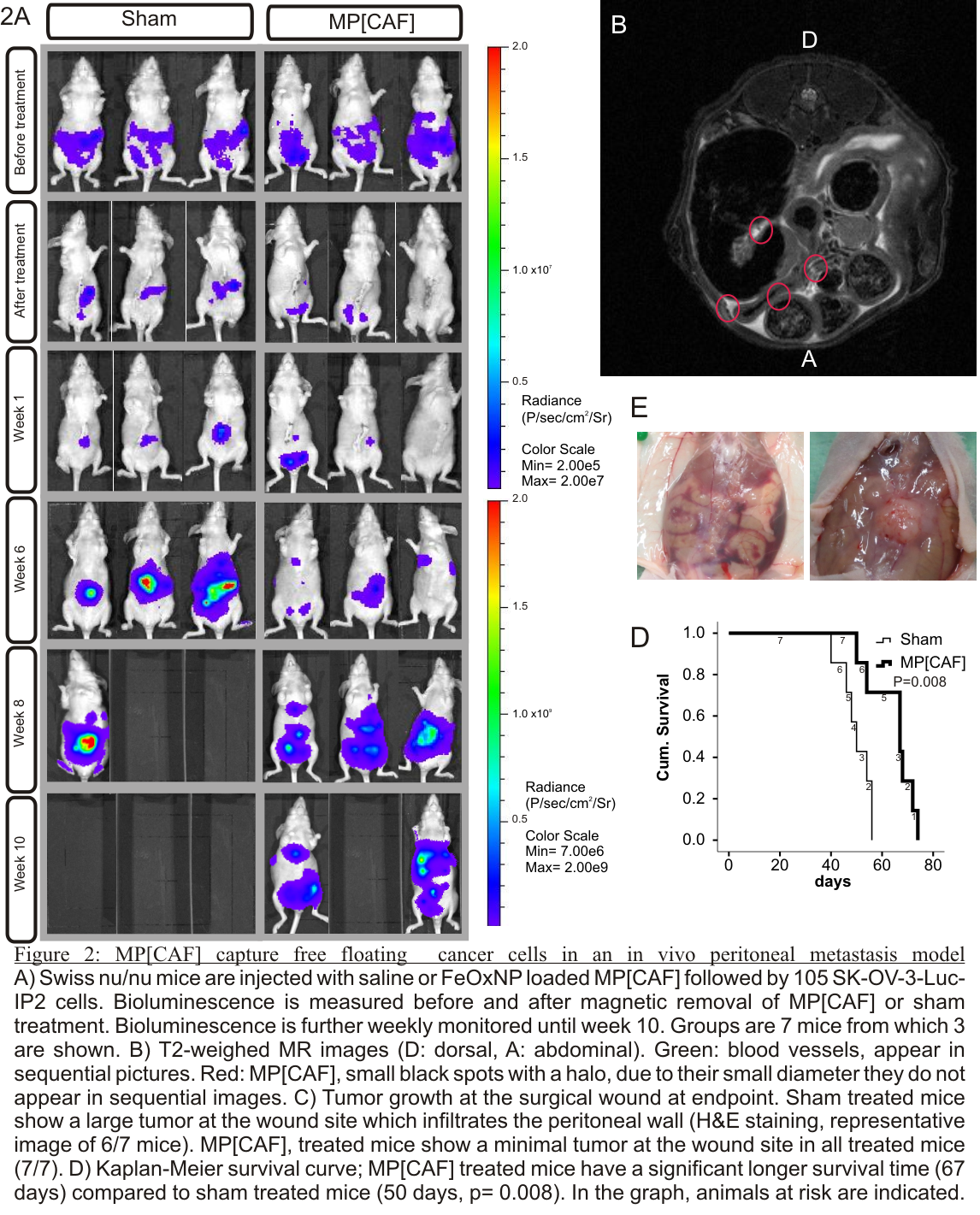
Conclusion: Biomimetics of the tumor-environment by encapsulating CAFs creates an ecological trap for disseminated cancer cells. The present results demonstrate the potential of ecological traps to prevent cancer metastasis.
Vlaamse Liga tegen Kanker; Stichting tegen Kanker; IWT
References:
[1] De Vlieghere et al. Biomaterials 2015, 54:148-157