Introduction: Tendons are load bearing tissues and substantially stronger along the load bearing direction than the direction transverse to the longer axis. Tendons also consist of parallel collagenous fibril along the longer axis of the tendon and tenoblasts are elongated (aspect ratio: 2-7) along the load bearing direction[1],[2]. It has been showed that matrix stiffness and topography determines the cellular morphology and fate[3]. Aligned topography of matrix resulted elongated and aligned cells in the direction of alignment. However, the roles of topographical factors (matrix anisotropy vs. matrix stiffness) in inducing the tenogenic differentiation are unknown. Therefore, the aim of this study was to manufacture collagen substrates with controlled stiffness and add anisotropy scenario to the substrates to mimic tendon topography and investigate the synergy of matrix stiffness and anisotropy in tenoinduction.
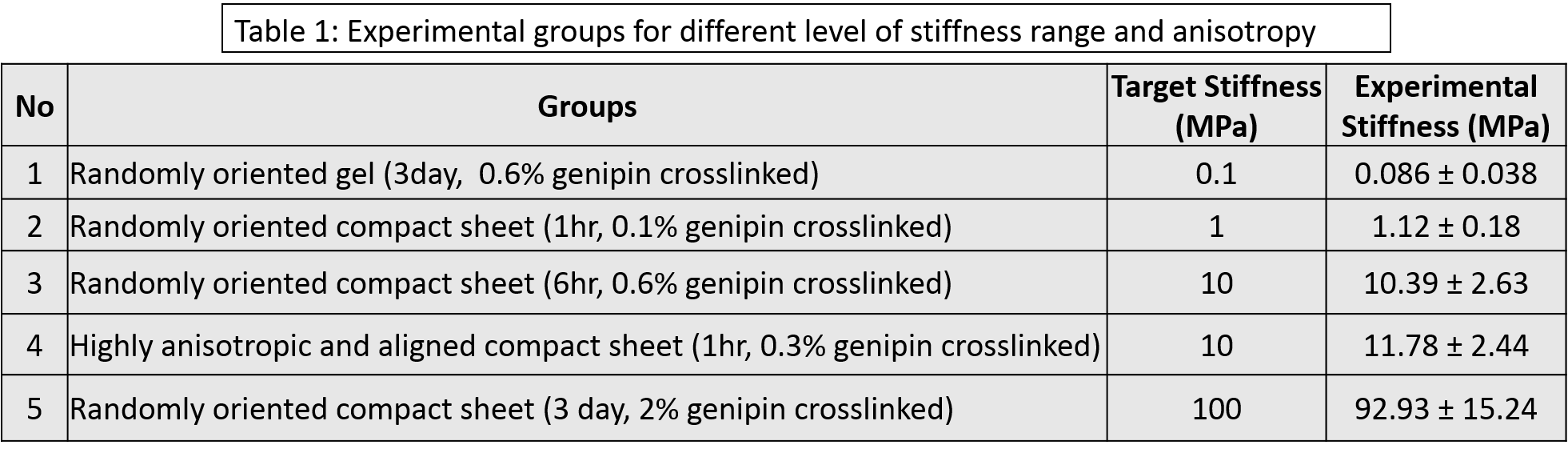
Methods: Electrochemically compact collagen sheet were made as described before[4],[5]. To check stiffness effect, four level of stiffness were chosen (0.1, 1, 10 and 100 MPa). These stiffness levels were attained by changing the crosslinking protocols [Table 1]. Crosslinking was carried out by genipin. For 10 MPa group, a highly anisotropic group was added to incorporate anisotropy scenario to the stiffness. The anisotropic sheet was generated by mechanical stretch of collagen sheet as described before[4]. The 10 MPa anisotropic group was crosslinked to a lesser extent than regular 10 MPa group [Table 1]. Samples were treated with per acetic acid ethanol solution after the cross linking to bleach out extra genipin which may keep crosslinking the samples. Samples were tested in tension until failure (10 mm/min) to check that sample groups attained the targeted stiffness. Mesenchymal stem cells (MSCs) were seeded on the 5 different sample groups at 20000 cells/ cm2 and cultured in regular media for 21 days. Tenogenic differentiation was assessed by RT-PCR at day 3, 14 and 21. Tendon related markers (Collagen I, Collagen III, and COMP); Tendon specific markers (Scleraxis, Thrombospondin) were assessed for tenogenic differentiation. Normalizing housekeeping gene was RPLP0. Fold change was calculated with respect to 0.1 MPa group at day 3. One-way analysis of variance (ANOVA) was performed for RT-PCR data and Tuckey’s post hoc analysis was performed for pairwise comparison. Significance was set at p<0.05.
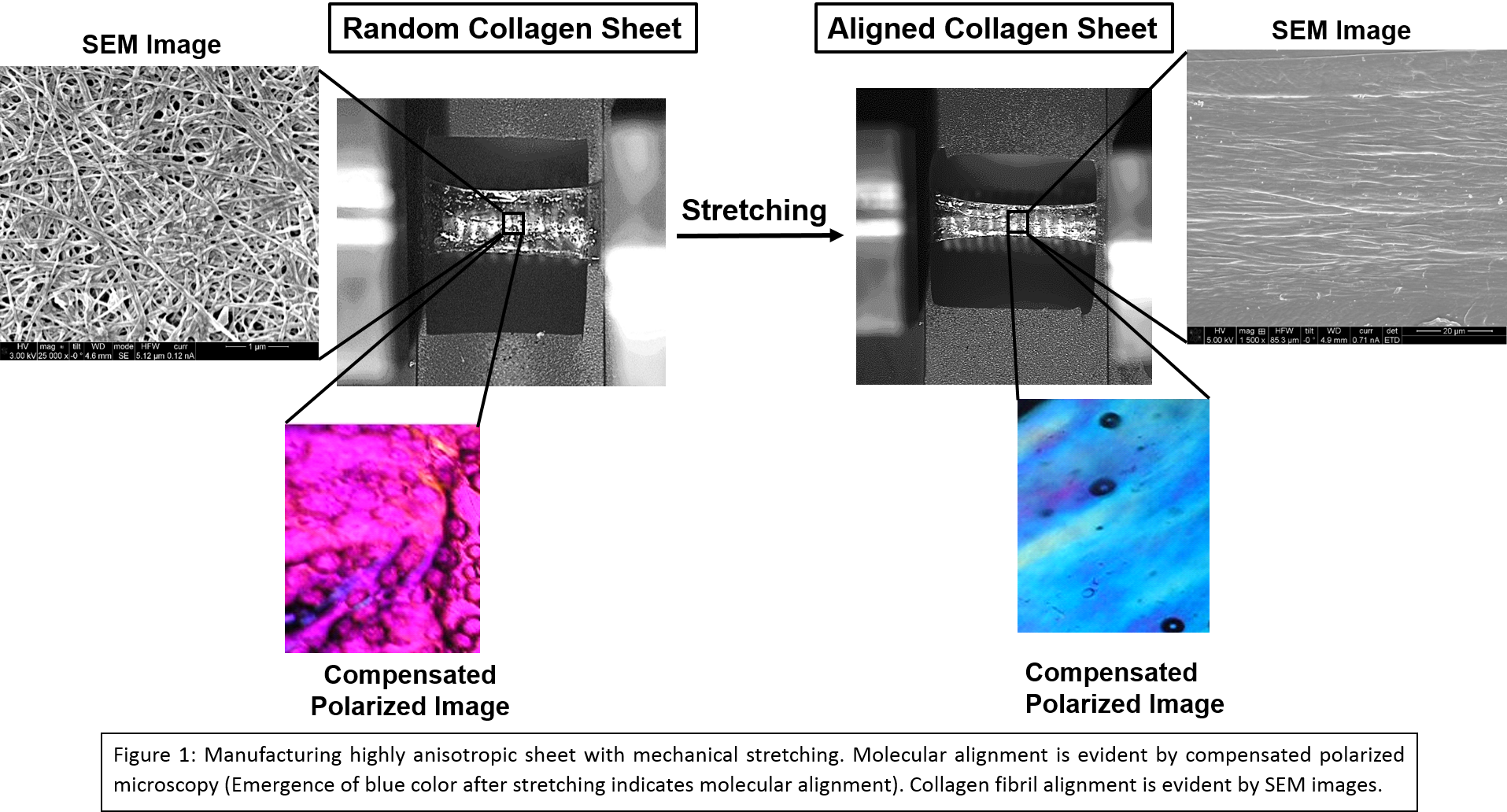
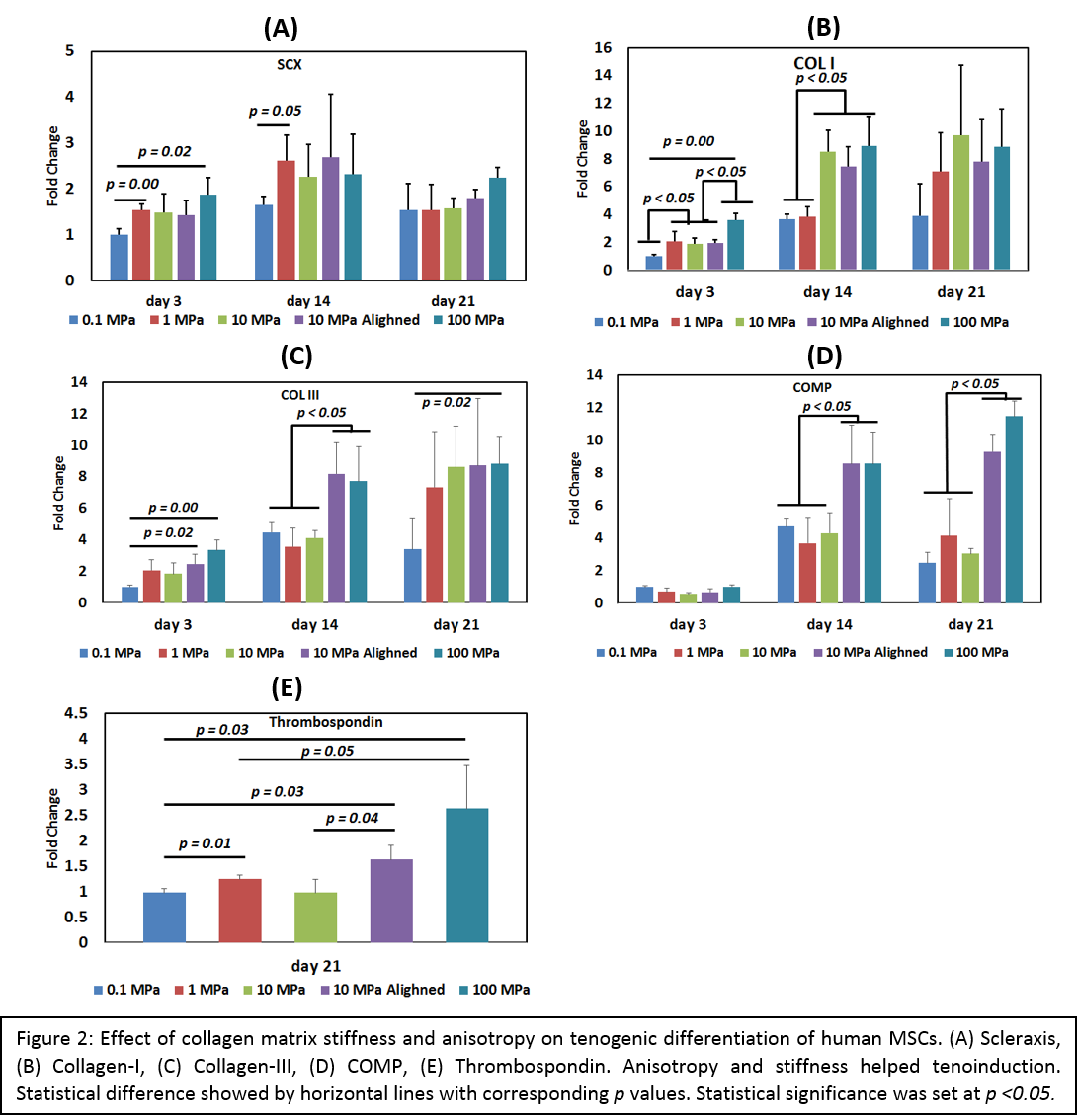
Results and Discussion: Mechanical test result indicated that the aforementioned crosslinking protocol was able to attain the targeted stiffness range [Table 1]. Mechanical stretching aligned the molecule as well as collagen fibril along the stretching direction [Fig. 1]. Early tendon marker SCX showed upregulation at day 3 for 100 MPa [Fig. 2A]. In case of COL I, stiffness expedited the expression [Fig. 2B]. Anisotropy did not have any effect on SCX and COL I expression at any time point at 10 MPa stiffness range [Fig. 2A, B]. At day 3 for COL III, 100 MPa was most favorable and 0.1 MPa was least favorable and as time passes the lower stiff substrate starts to pick up differentiation [Fig. 2C]. For COMP, at day 3 there was no change in expression among the groups whereas at day 14 and 21, 100 MPa group showed higher expression than 0.1-10 MPa range [Fig. 2D]. For both COL III and COMP, anisotropy increase the expressions and 10 MPa anisotropic group showed similar expression as 100 MPa [Fig. 2C, D]. Alignment and stiffness favored thrombospondin expression [Fig. 2E]. Both the stiffness and anisotropy have effect on tenoinduction. In most cases, 100 MPa and anisotropic substrate showed early sign of tenogenic marker expression whereas 1-10 MPa groups performed similarly. In some cases, 0.1-10 MPa groups picked up tenoinduction at later time points.
Conclusion: Over all, stiffness favored tenoinduction. Anisotropic sheet were stronger along the longitudinal direction and collagen fibril are aligned along the stiffer direction. Therefore, this group was able to mimic tendon topography in some extent and showed increased tenoinduction.
References:
[1] Moore MJ & De Beaux A (1987) A quantitative ultrastructural study of rat tendon from birth to maturity. Journal of Anatomy 153:163-169.
[2] Kannus P (2000) Structure of the tendon connective tissue. Scand J Med Sci Sports 10(6):312-320.
[3] Engler AJ, Sen S, Sweeney HL, & Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677-689
[4] Islam A, Cotey S, Younesi M, Akkus O "Tuning of Stiffness Anisotropy in Collagen Sheets by Planar Stretch" Society for Biomaterials, 2014
[5] Islam A, Chapin K, Younesi M, Akkus O" Computer aided biomanufacturing of mechanically robust pure collagen meshes with controlled macroporosity" Biofabrication, 2015