Introduction: Metastasis, the spread of cancer to secondary sites, accounts for 90% of cancer-related deaths. Bone metastasis has the highest rate of morbidity in breast cancer patients, and results in tumors that disrupt bone remodeling and production of immune cells. Many hypothesize that metastasis depends on both the cancer cell and the properties of the secondary tissue, but most research in breast cancer spread to bone neglects the contributions of the bone marrow tissue[1]. Here, we have designed a polymer hydrogel that mimics some aspects of human bone marrow tissue to better study breast-to-bone metastasis.
Materials and Methods: Our hydrogel consists of a 4-arm PEG-maleimide that encapsulates cells in a 3D microenvironment (Figure 1a). The mechanics of our hydrogel are tuned by adjusting polymer wt%. Mechanics of intact porcine marrow were measured using rheology, indentation, and cavitation rheology[2]. The hydrogel is biochemically modified by crosslinking with 7 peptide sequences that degrade in response to cell-secreted enzymes, and by incorporating 13 peptides representing cell-binding sites. Immunohistochemistry and RNA-seq data was used to identify these biochemical features in human bone marrow[3]. Final peptide concentrations will be determined using tandem mass tags to label and quantify bone marrow, lung, and brain tissue for analysis with liquid chromatography mass spectrometry (LC-MS).
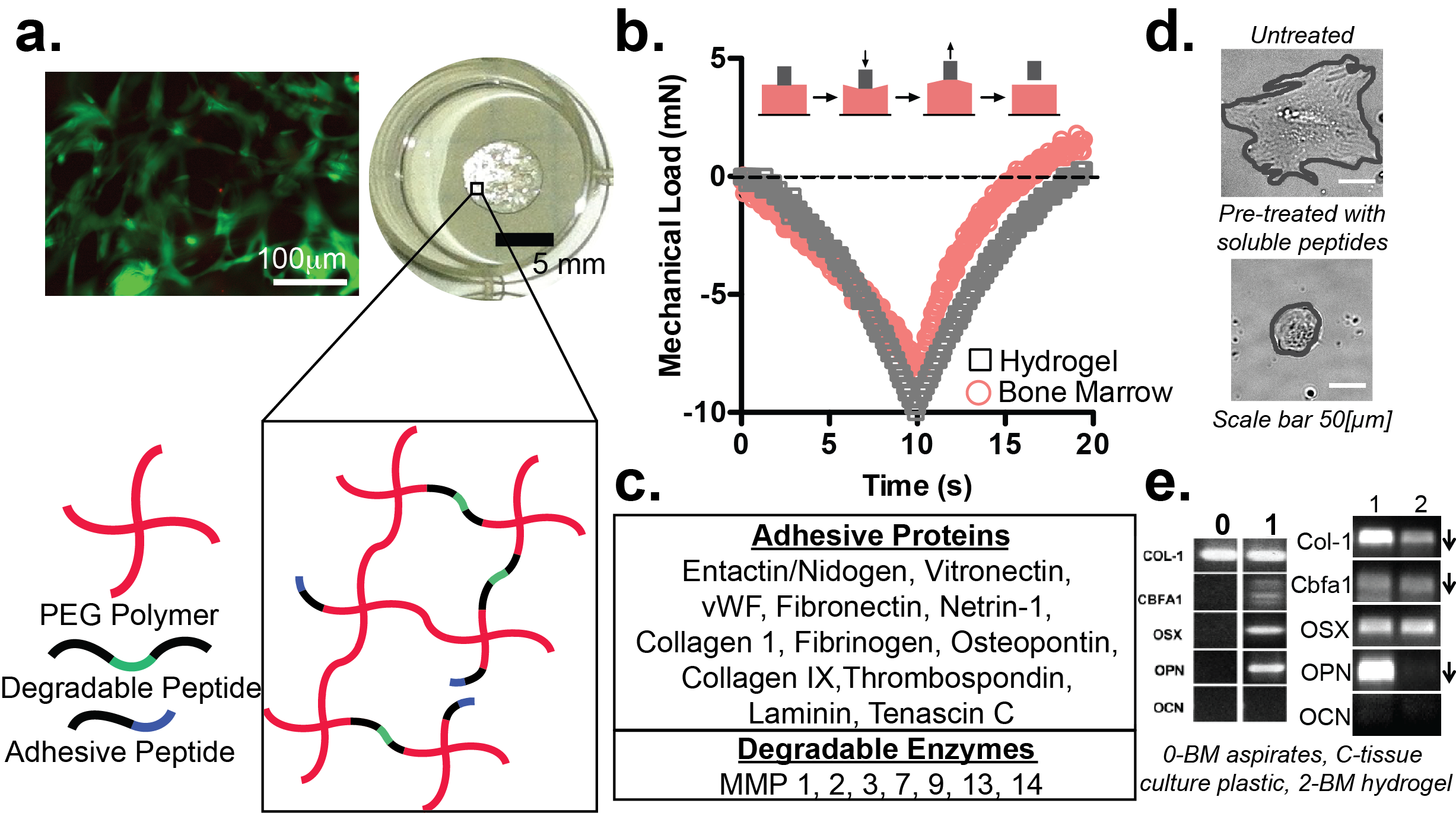
Figure 1. a. Our bone marrow hydrogel (bottom, top right) maintains high cell viability in 3D culture (hMSCs after 14 days, top left). b. Marrow and our hydrogel are benign elastic materials upon compression. c. Biochemical features represented in our tissue mimic. d. Cells pre-treated with soluble peptides spread less indicating peptide binding (representative images). e. RT-PCR of hMSCs encapsulated in our hydrogel (2) are more similar to native marrow (0) than hMSCs on plastic (1), (BM is bone marrow)[4].
Results and Discussion: Marrow and our hydrogel are benign elastic materials, so we were able to match hydrogel mechanics to the average Young’s modulus of porcine marrow, 4.4±1.0 kPa at physiological temperature (Figure 1b)[2]. We identified 20 biochemical features, using the ProteinAtlas, in human marrow that are represented as peptide sequences in our system (Figure 1a, c). A competitive binding assay validated that hMSCs adhere to each of these cell-binding peptides (Figure 1d). Preliminary, we found that hMSCs encapsulated in a hydrogel with mechanics tuned to marrow and coupled with some biochemical features (4 cell binding, 3 degradable) were more similar to hMSCs in native marrow tissue than on plastic (Figure 1e)[4]. We hypothesize that including all the bone marrow peptides at native concentrations, identified through LC-MS on human marrow tissue, will push hMSCs expression closer to hMSCs in tissue. Our final system will be used to screen for phenotypes across 4 patient hMSCs and 5 breast cancer cell lines to identify conditions that increase breast cancer displacement and movement.
Conclusion: We have fundamentally quantified the mechanical and proteomic properties of bone marrow tissue. This knowledge was used to design an in vitro bone marrow model of the extracellular matrix. Using this system we can easily isolate phenotypes between cancer cells, local cells, and bone marrow tissue that could be implicated in successful breast cancer cell adhesion and proliferation in secondary tissues.
SRP is a Pew Biomedical Scholar supported by the Pew Charitable Trusts.; This work was funded by an NIH New Innovator award (1DP2CA186573-01).
References:
[1] Kang, et al, Cancer Cell. 2003.
[2] Jansen et al, JMBBM. 2015.
[3] Uhlén, et al, Science, 2015.
[4] Gronthos et al, J. Cell Science, 2003.