Recent years have seen significant progress in two distinct tissue engineering fields: 3D biomaterial printing and processing and utilization of decellularized extra cellular matrix (dECM). However, there are still significant needs that can be addressed by increasing the 3D biomaterial palette[4] as well as developing new and effective means to process and utilize tissue-specific dECM[2]. We present a new, efficient, and highly versatile method for creating a broad range of tissue-specific simple and complex constructs through integration of novel material casting and 3D-printing technologies recently developed by the authors[1],[3]. 2D-castable and 3D-printable, liquid-based inks (inks) are synthesized through combination of several organic solvents, biocompatible elastomer (30 vol.% solids loading), and particulate material (70 vol.% solids loading)[1],[3]. In this instance, the particulate material is tissue specific dECM, created by decellularizing porcine and bovine tissues and organs followed by lyophilization and mechanical milling.
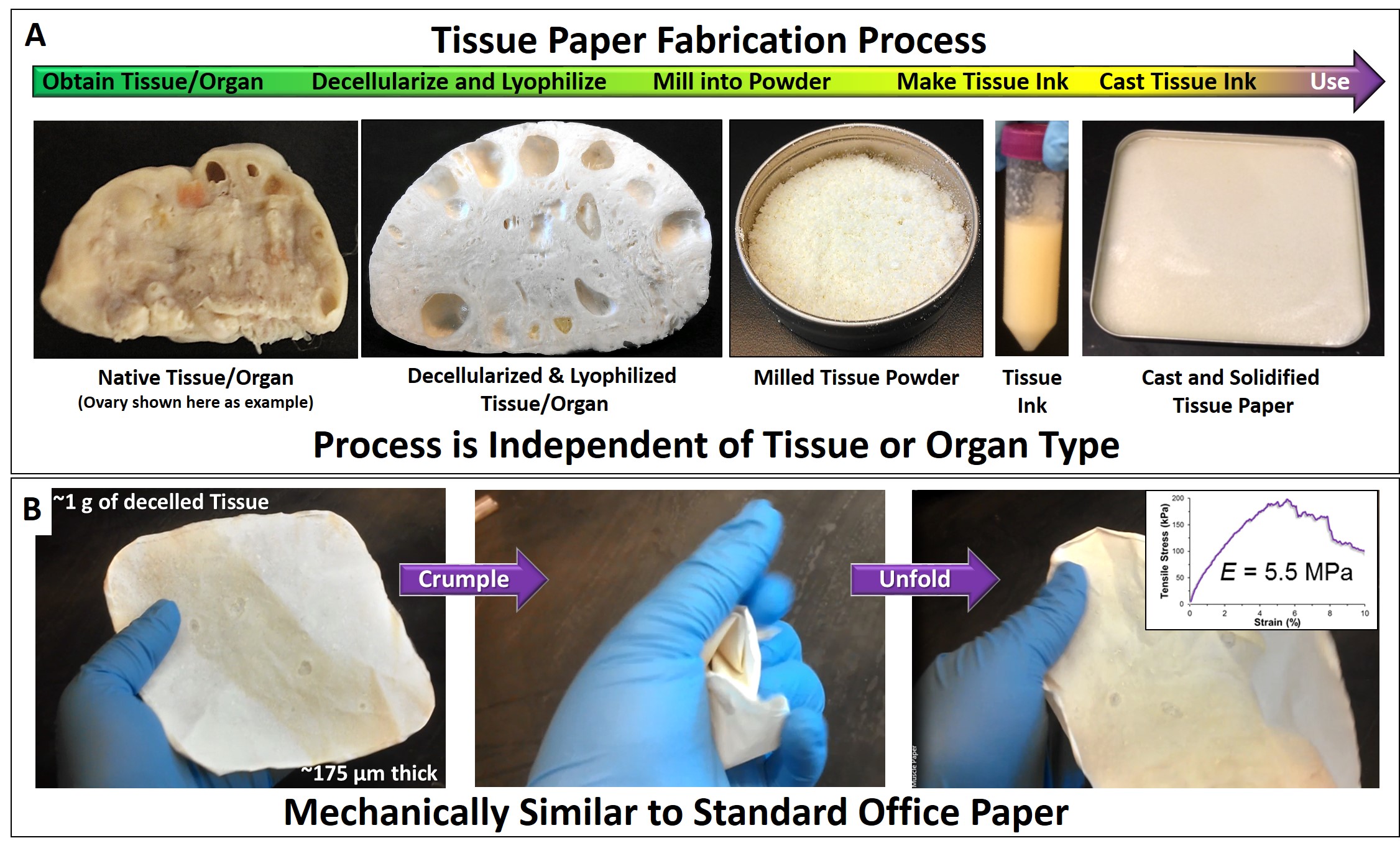
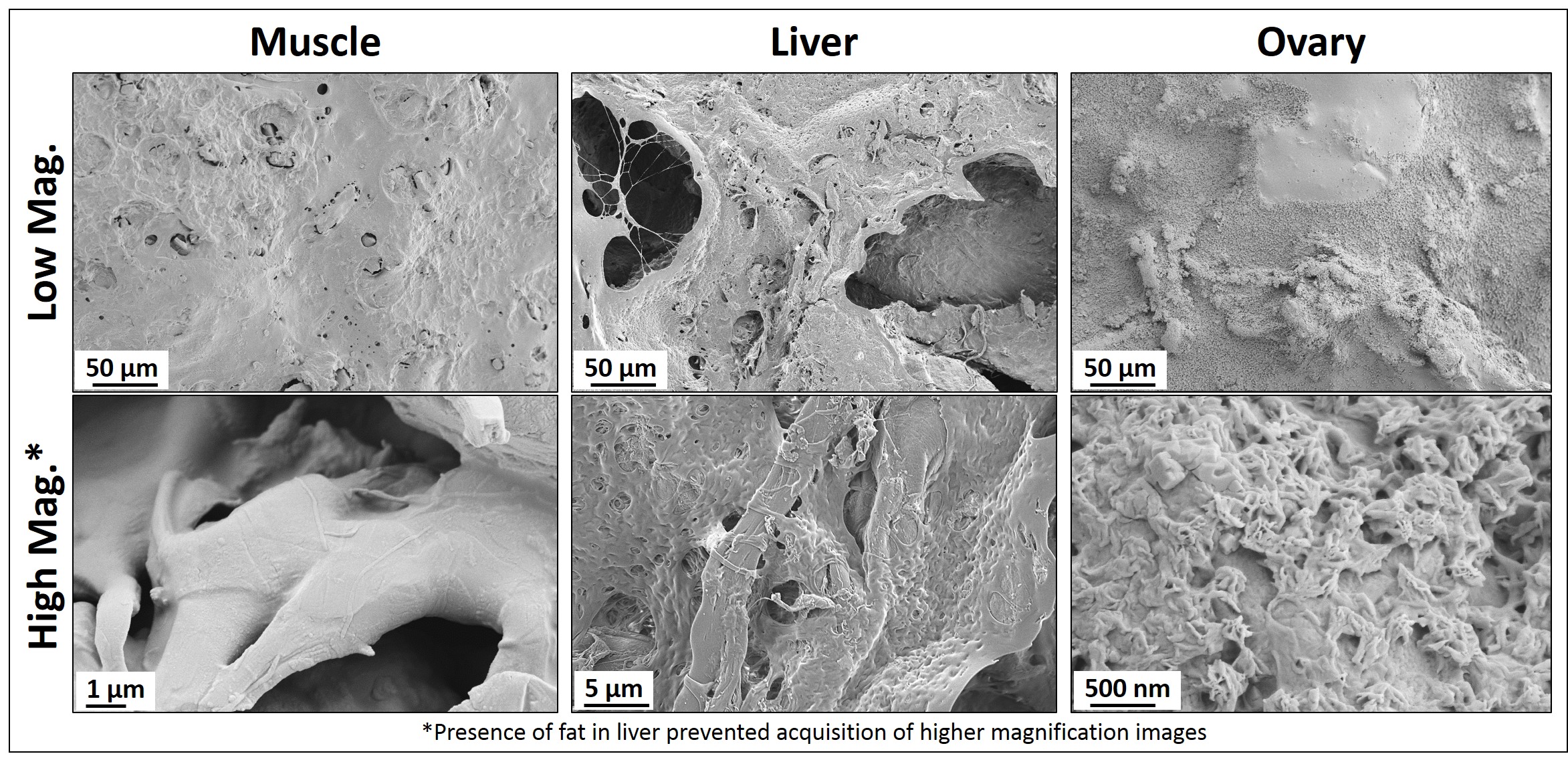
Once incorporated within the ink formulation via simple mixing, the ink may be immediately cast into a container (Fig. 1A). After several minutes, the organic solvents evaporate and “tissue paper” results; so named due to its thickness of 100-200 µm and mechanical and handling properties closely resembling common office paper. Similar to standard paper, the dECM can be cut, folded, crumpled (Fig.1B), rolled, and even sutured to soft tissues. Although the fabrication process and, to a lesser extent, resulting mechanical properties (Fib. 1B inset) are independent of the tissue or organ being utilized, compositional analyses and structural evaluation via scanning electron microscopy (SEM) reveal that papers derived from distinct tissues are themselves distinct (Fig. 2).
To create thicker or more complex tissue constructs comprised of the same or several distinct dECM types, the paper can be stacked (Fig. 3A). Small solvent volumes, similar to those used in creating the initial ink, can be applied to individual layers and subsequently fused together. Through this process, thick, multi-layered single or multi-tissue-type constructs can be created by fusing together dECM paper of the same or differing type, respectively. Expanding on the versatility of this planar system, these tissue papers can be integrated with 3D-printed biomaterials derived from the exact same particle-based ink system including the highly osteogenic Hyperelastic Bone[1] (HB) and highly neurogenic 3D-Graphene[3] (3DG) to create three-dimensional, porous multi-functional constructs (Fig.3B). Due to the similarity in composition, these extruded 3D-inks instantly fuse with tissue papers, acting as printing substrates, upon contact (Fig. 3B and 3C). Additionally, independently 3D-printed materials derived from this ink system can be manually fused to single or stacked tissue papers through application of solvent at points of contact (Fig. 3C).
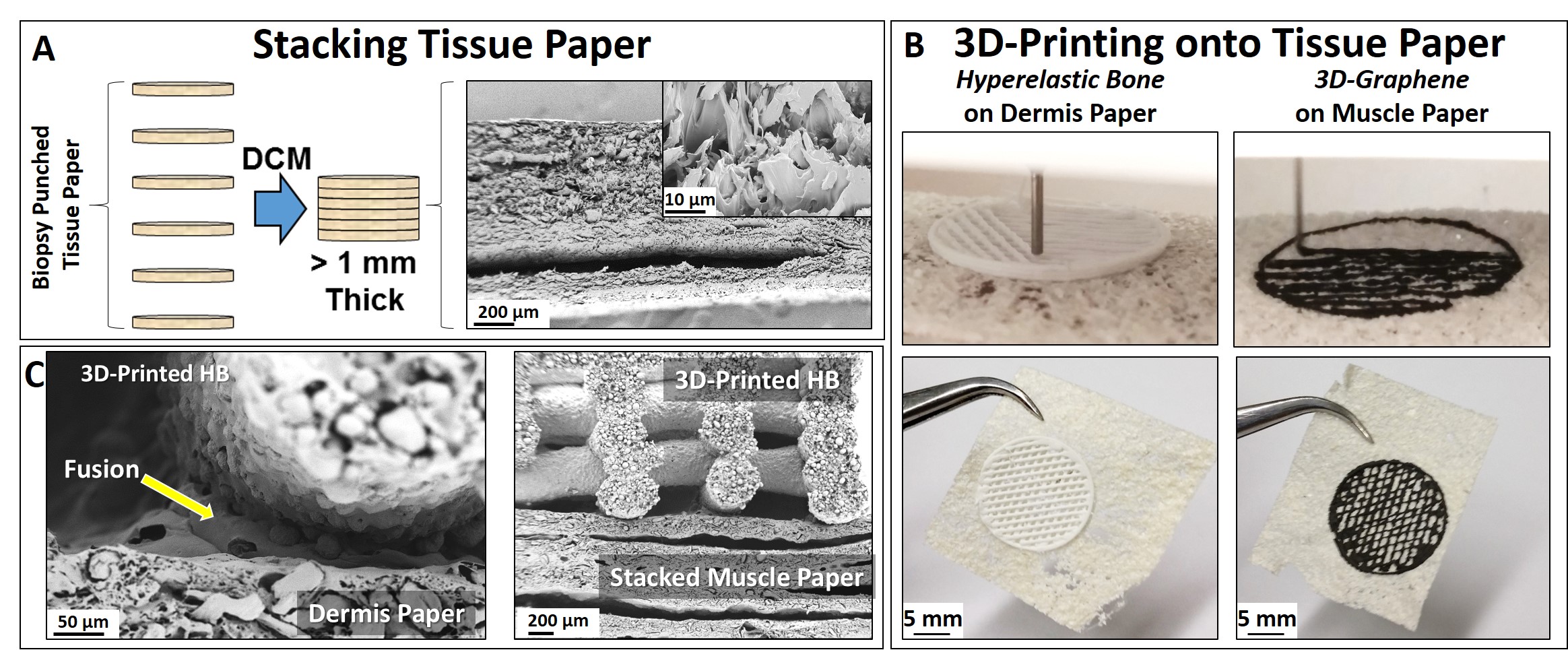
Utilizing this process, we illustrate that complex, 3D-printed constructs can be directly printed onto the tissue paper, ultimately creating biomaterial structures mimetic of bone-nerve-muscle-adipose-dermis complex tissue structures. Additionally, we present methods for fabricating functional tissue papers from porcine lung, liver, heart, kidney, dermal, muscle, and adipose tissues, present their mechanical and microstructural properties, and in vitro capacity to influence stem cell differentiation and function. Brief discussion will be given to initial in vivo tissue regeneration results.
Adam Jakus was supported by a post-doctoral fellowship through The Hartwell Foundation
References:
[1] A.E. Jakus, R.N. Shah et al. "Hyperelastic Bone: Discovering New Materials Through 3D-Printing". Biomaterials. Under Review. 2015
[2] M.L. Laronda, A.E. Jakus, et al. "Initiation of puberty in mice following decellularized ovary transplant". Biomaterials. 50. 2015.
[3] A.E. Jakus, R.N. Shah et al. "Three-Dimensional Printing of High-Content Graphene Scaffolds for Electronic and Biomedical Applications". ACS Nano. 9(4), 2015. Cover.
[4] A.E.Jakus, A.L. Rutz, R.N. Shah. "Advancing the field of 3D biomaterial printing". Biomedical Materials. 10th Anniversary Special Issue. In Press.