Introduction: Bone fractures and non-union defects often require surgical interventions where biomaterials are used to repair the injury. In the US, nearly 112,000 orthopedic device infections occur annually[1], motivating the development of bifunctional materials that promote regeneration and prevent failure due to infection.
Our lab has recently developed a poly(ethylene glycol) (PEG)-based hydrogel to regenerate bone in a critical-sized radial defect in a mouse by delivering BMP-2 and integrin-specific peptides[2]. We have extended this model to evaluate the antimicrobial properties of these hydrogels. Bacteriophages are viruses specific to bacteria causing lysis. Upon host infection, the phage amplifies and propagates throughout the infection site, but is self-limited in that it cannot infect eukaryotic cells, providing an on-demand response to pathogens. The objective of this study is to evaluate the efficacy of bacteriophage presenting hydrogels in an infection model of bone repair.
Materials and Methods: PEG-mal hydrogel synthesis: PEG-maleimide hydrogels[3] were functionalized with the collagen mimetic peptide GFOGER and cross-linked with protease degradable peptides.
Bacteriophage release: A bacteriophage mixture of ɸPaer4, ɸPaer14, ɸPaer22, and ɸW2005A was labeled with AlexaFluor 488 and encapsulated in PEG hydrogels cross-linked with VPM, GPQ-W, or PEG-dithiol. Hydrogels were swollen in DPBS or 2U/mL collagenase and fluorescence of the swelling supernatant was measured.
Bacteriophage delivery in vivo: A 2.5mm radial defect in male C57/B6 mice was created and hydrogels containing Pseudomonas aeruginosa PsAer-9 pSEVAplaxA, and a mixture of ɸPaer4, ɸPaer14, ɸPaer22, and ɸW2005A, were implanted in the defect (Fig. 2A). At 1 week, implants were explanted, and assayed for viable bacteria and bacteriophage.
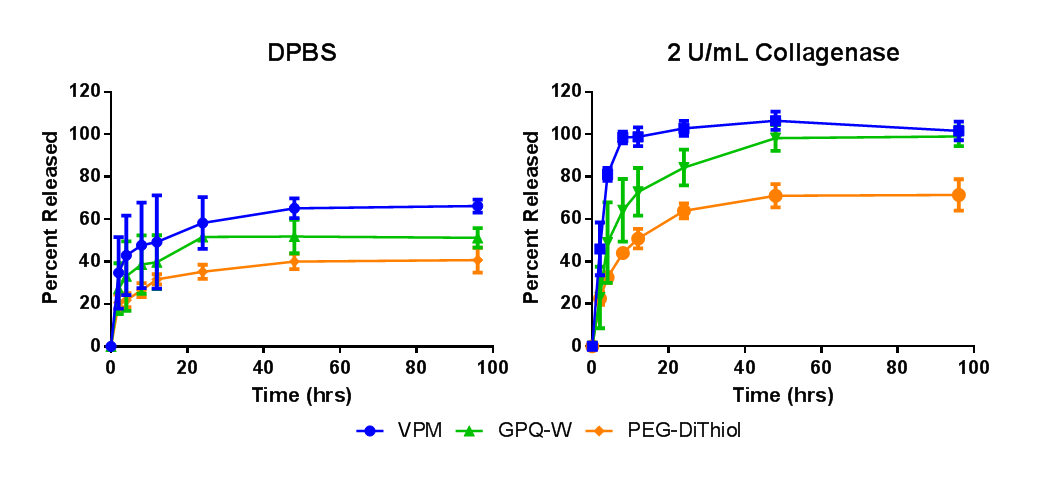
Figure 1: Percent release of fluorescently labeled phage from hydrogels swollen in A) DPBS and B) 2 U/mL collagenase.
Results and Discussion: Figure 1A demonstrates that phage is retained in the hydrogel when swollen in a protease-free medium. The addition of collagenase (Fig. 1B) promotes the degradation of the hydrogel and subsequent release of bacteriophage. Modification of the cross-linker allows for the realization of fast (VPM), slow (GPQ-W), and non-degradable (PEG-dithiol) phage release.
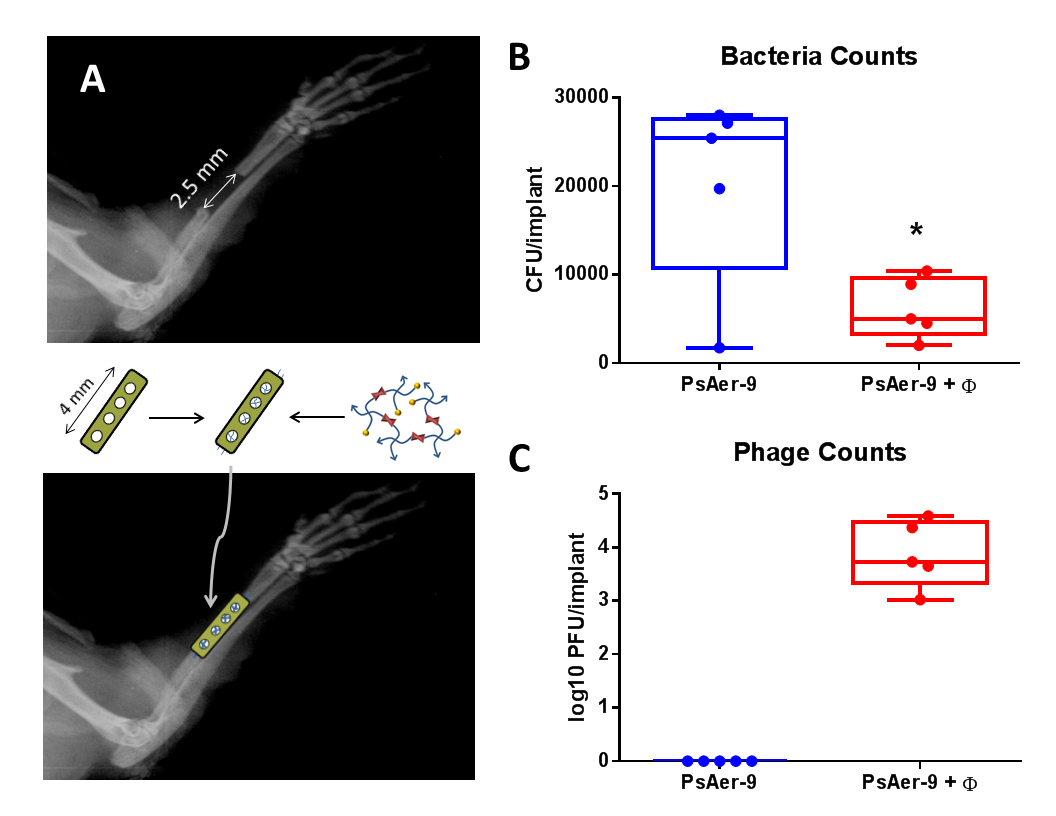
Figure 2: A) Mouse radial defect schematic. Viable B) PsAer-9 and C) Phage recovered at 1 week (* p < 0.05)
Hydrogels cross-linked with VPM were loaded with PsAer-9 or PsAer-9 and a mixture of phage and implanted into a mouse radial segmental defect (Fig. 2A). One week after implantation, viable bacteria (Fig. 2B) and viable bacteriophage (Fig. 2C) were recovered. A significantly lower (p<0.05) amount of bacteria was recovered in the phage treated group compared to control (Fig. 2B) indicating that phage therapy reduces infection. Bacteriophage was recovered from all phage-treated samples (Fig. 2C), demonstrating that phage persists in vivo in response to infection.
Conclusion: Bacteriophage release can be controlled by modifying the hydrogel cross-linker. Bacteriophage remains active within the infection site demonstrating the on-demand response of phage therapy. Importantly, phage therapy reduces infection, supporting the feasibility of phage based therapeutics. Further studies will focus on optimizing the antimicrobial properties of the hydrogel to further reduce infection while also promoting bone regeneration.
Funding provided by S2013/MIT-2807 (MAP) and NIH grants R01 AR062368 and R01 AR062920 (AJG). We thank Rodney Donlan for bacteria and bacteriophage samples and V. de Lorenz for plasmids.
References:
[1] Nair MB, Kretlow JD, Mikos AG, and Kasper FK. Infection and tissue engineering in segmental bone defects--a mini review. Curr Opin Biotechnol 2011; 22:721-5.
[2] Shekaran A, Garcia JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, and Garcia AJ. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014;
[3] Phelps EA, Enemchukwu NO, Fiore VF, Sy JC, Murthy N, Sulchek TA, Barker TH, and Garcia AJ. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv Mater 2012; 24:64-70, 2.