Introduction: Nanosized graphene oxide (NGO) has been widely investigated for biomedical applications. In particular, NGO has a high photothermal effect under near-infrared (NIR) irradiation due to their effective light-to-heat conversion capability[1].
Hyaluronic acid (HA) has been regarded as one of the best biopolymers in terms of safety issues. The structural hydrophobic domain of HA can facilitate its penetration into skin tissues[2]. Especially, tumor cells are known to have overexpressed HA receptors like cluster determinant 44 (CD44). Here, we report transdermal NGO-HA conjugates for photothermal ablation therapy of melanoma skin cancer using a NIR laser as schematically shown in Figure 1.
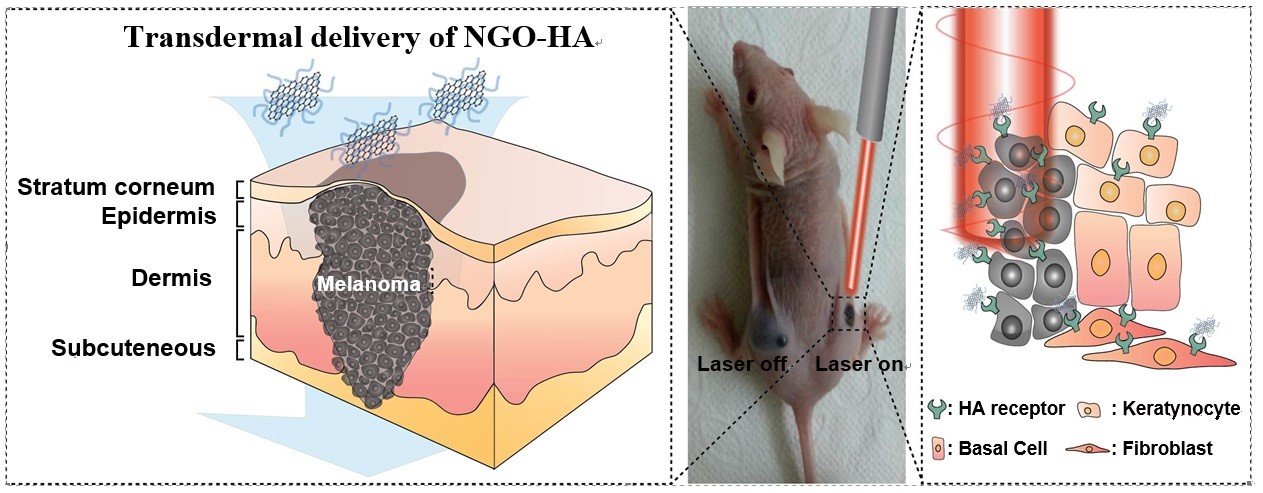
Materials and Methods: Carboxylated NGO was prepared by the tip sonication and the activation of microsized GO solution with chloroacetic acid. Then, hexamethylene diamine-HA was synthesized and conjugated to NGO-COOH for the preparation of NGO-HA by amide bond formation using the EDC chemistry. The synthesis of NGO-HA was confirmed by AFM, TEM, FT-IR, and Raman spectroscopic analyses. In vivo transdermal delivery of dye labeled NGO-HA (NGO-HA-Hilyte647) was visualized by confocal microscopy and IVIS system. Tumor growth was monitored for 7 days following caspase-3 ELISA for the analysis of tumor apoptosis.
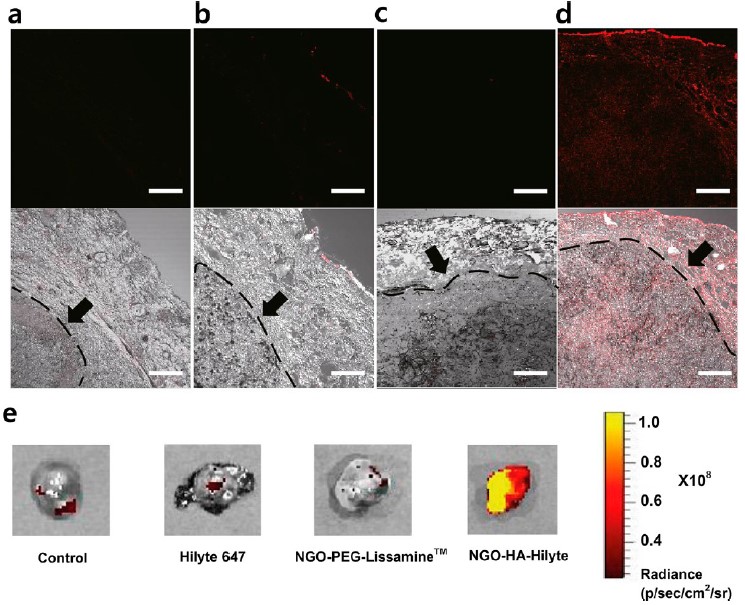
Results: We carried out ex vivo bioimaging to visualize the transdermal delivery of NGO-HA-Hilyte647 to SKH-1 mice inoculated with melanoma cells. NGO-HA-Hilyte647 was significantly delivered through the damaged cancerous skin barrier (Figure 2d) while PBS (Figure 2a), dye only (Figure 2b) and dye labeled NGO-PEG (Figure 2c) showed no significant signals. The red fluorescence of NGO-HA-Hilyte647 was observed in every site of the skin tumor tissue. The fluorescence could be detected from the top and even from the bottom of the dissected 5mm long tumor tissues (Figure 2e).
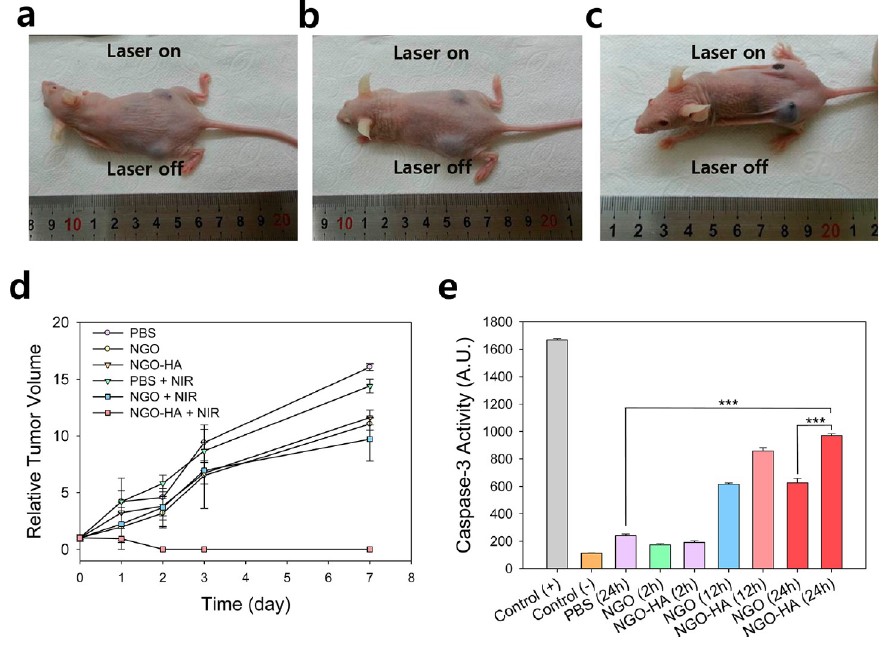
The photothermal ablation therapy was carried out using PBS, NGO and NGO-HA. The treatment with PBS and NGO showed no significant tumor ablation regardless of NIR irradiation (Figure 3a,b). In the case of NGO-HA, tumor tissues was completely ablated by the photothermal therapy with NIR irradiation (Figures 3c). Also, there was no recurrence of tumorigenesis for 7 days (Figure 3d). The caspase-3 activity after treatment with NGO-HA in the presence of NIR irradiation was statistically higher than that with PBS or NGO in 24 h (Figure 3e).
Discussion: The effective transdermal delivery of NGO-HA can be explained by structure of tumor microenvironment. The tumor growth under the skin might form leaky structures around tumor and increase the skin permeation, rendering the effective transdermal delivery of NGO-HA[3]. Diffusion of NGO-HA through the interstitial tumor space might enhanced deep penetration of NGO-HA into tumor. Highly expressed HA receptors in tumor tissues also facilitated the transdermal delivery of NGO-HA.
Conclusion: We successfully developed a transdermal NGO-HA conjugate for photothermal ablation therapy of melanoma skin cancer using a NIR laser. Bio imaging of skin cancer clearly showed the effective transdermal delivery of NGO-HA. The NIR irradiation resulted in complete ablation of tumor with no recurrence of tumorigenesis. The antitumor photoablation effect was confirmed by ELISA for caspase-3 activity. This system is expected to be applied for various skin related diseases treatments.
References:
[1] H.S. Jung et al, ACS Nano, 2014, 8, 260
[2] J. A. Yang et al, Biomaterials, 2012, 33, 5947
[3] H. S. Jung et al, Nanomedicine, 2014, 9, 743